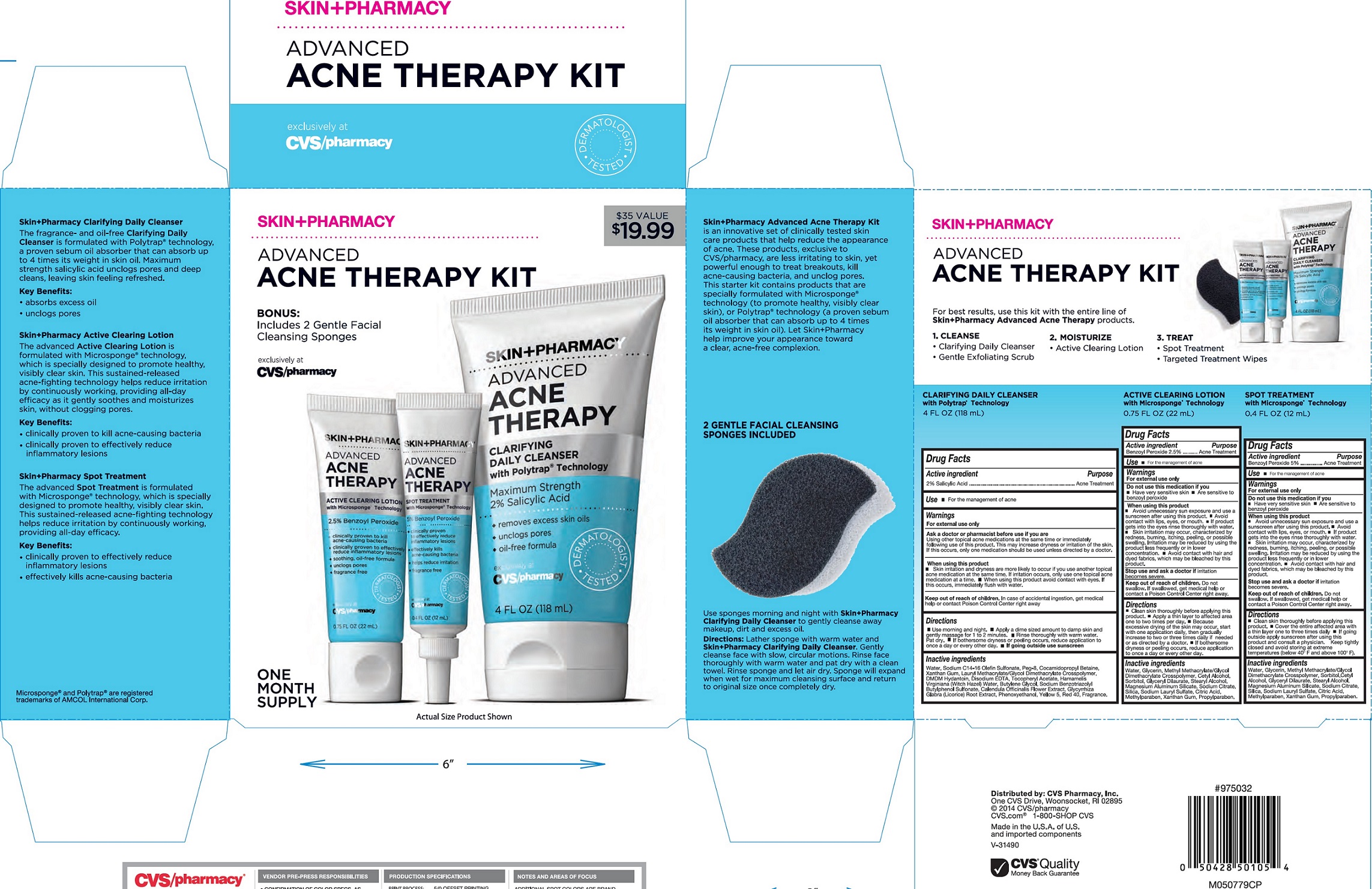
CVS ADVANCE ACNE THERAPY KIT- salicylic acid, benzoyl peroxide
Diversified Global Technologies, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Advance Acne Therapy Clarifying Daily Cleanser with Polytrap Technology
Active ingredient
2 % Salicylic Acid
Use
- For the management of acne
Warnings
For external use only
Ask a doctor or pharmacist before use if you are
Using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a docotor.
When using this product
- Skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irrtiation occurs, only use topical acne medication at a time.
- When using this product avoid contact with eyes. If this occurs, immediately flush with water.
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact Poison Control Center right away
Directions
- Use morning and night.
- Apply a dime sized amount to damp skin and gently massage for 1 to 2 minutes.
- Rinse thoroughly with warm water. Pat dry.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- If going outside use sunscreen
Inactive ingredient
Water, Sodium C14-16 Olefin Sulfonate, Peg-8, Cocamidopropyl Betaine, Xanthan Gum, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, DMDM Hydantoin, Disodium EDTA, Tocopheryl Acetate, Hamamelis Virginiana (Witch Hazel) Water, Butylene Glycol, Sodium Benzotriazolyl Butylphenol Sulfonate, Calendula Officinalis Flower Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Phenoxyethanol, Yellow 5, Red 40, Fragrance.
CVS Advance Acne Therapy Spot Treatment with Microsponge Technology
Active ingredient
Benzoyl Peroxude 5%
Use
- For the management of acne
Warnings
For external use only
Do not use this medication if you
- Have very sensitive skin
- Are senstive to benzoyl peroxide
When using this product
- Avoid unnecessary sun exposure and use a sunscreen after using this product.
- Avoid contact with lips, eyes, or mouth.
- If product gets into the eyes rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
Stop use and ask a doctor if
irritation becomes severe.
Keep out of reach of children.
Do not swallow. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily
- If going outside apply sunscreen after using this product and consult a physician. Keep tightly closed and avoid storing at extreme temperatures (below 40° F and above 100° F).
Inactive ingredients
Water, Glycerin, Methly Methacrylate/Glycol Dimethacrylate Crosspolymer, Sorbitol, Cetyl Alcohol, Glyceryl Dilaurate, Stearyl Alcohol, Magnesium Aluminum Silicate, Sodium Citrate, Silica, Sodium Lauryl Sulfate, Citric Acid, Methylparaben, Xanthan Gum, Propylparaben.
CVS Advance Acne Therapy Active Clearing Lotion with Microsponge Technology
Active ingredient
Benzoyl Peroxude 2.5%
Use
- For the management of acne
Warnings
For external use only
Do not use this medication if you
- Have very sensitive skin
- Are senstive to benzoyl peroxide
When using this product
- Avoid unnecessary sun exposure and use a sunscreen after using this product.
- Avoid contact with lips, eyes, or mouth.
- If product gets into the eyes rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
Stop use and ask a doctor if
irritation becomes severe.
Keep out of reach of children.
Do not swallow. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean skin thoroughly before applying this product.
- Apply a thin layer to affected area one to two times per day.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
Water, Glycerin, Methly Methacrylate/Glycol Dimethacrylate Crosspolymer, Cetyl Alcohol, Sorbitol, Glyceryl Dilaurate, Stearyl Alcohol, Magnesium Aluminum Silicate, Sodium Citrate, Silica, Sodium Lauryl Sulfate, Citric Acid, Methylparaben, Xanthan Gum, Propylparaben.
Package Labeling:
Package Labeling:

Package Labeling:

Package Labeling:




