vusion (miconazole nitrate, zinc oxide and white petrolatum) ointment
[Barrier Therapeutics, Inc.]
DESCRIPTION
VUSION Ointment contains the synthetic antifungal agent, miconazole nitrate (0.25%), zinc oxide (15%) and white petrolatum (81.35%) for topical use.
The chemical name of miconazole nitrate is 1-[2, 4-dichloro-ß-{(2,4-dichlorobenzyl)oxy} phenethyl] imidazole mononitrate with empirical formula C18H14Cl4N2O•HNO3 and molucular weight of 479.15. The structural formula of miconazole nitrate is as follows:
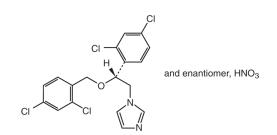
The zinc oxide has an empirical formula of ZnO and a molecular weight of 81.39.
The white petrolatum, which is obtained from petroleum and is wholly or nearly decolorized, is a purified mixture of semisolid saturated hydrocarbons having the general chemical formula CnH2n+2. The hydrocarbons consist mainly of branched and unbranched chains. White petrolatum contains butylated hydroxytoluene (BHT) as stabilizer.
Each gram of VUSION Ointment contains 2.5 mg of miconazole nitrate USP, 150 mg of zinc oxide USP, 813.5 mg of white petrolatum USP containing butylated hydroxytoluene, trihydroxystearin and Chemoderm® 1001/B fragrance. 1
VUSION Ointment is a smooth, uniform, white ointment.
- 1
- Chemoderm®is a registered trademark of Firmenich Inc.
CLINICAL PHARMACOLOGY
Pharmacokinetics:
The topical absorption of miconazole from an ointment containing 0.25% miconazole nitrate, 15% zinc oxide and 81.35% white petrolatum was studied in male and female infants (n=18) with diaper dermatitis ranging in age from 1 month to 12 months. After application at every diaper change for 7 days, the plasma concentrations of miconazole were nondetectable (<1 ng/mL) in the majority (15/18) of patients and ranged from 3 to 3.8 ng/mL in the other 3 remaining patients.
Microbiology
The miconazole nitrate component in this product has been shown to have in vitro activity against Candida albicans, an organism that is associated with diaper dermatitis. The activity of miconazole nitrate against C albicans is based on the inhibition of the ergosterol biosynthesis in the cell membrane. The accumulation of ergosterol precursors and toxic peroxides results in cytolysis of the cell. In vitro minimal inhibitory concentration (MIC) test results for C albicans isolates obtained from treatment failures in clinical Study 1 (see CLINICAL STUDIES) does not appear to indicate that resistance to miconazole nitrate was the reason for treatment failure. The clinical significance of the in vitro activity of miconazole nitrate against C albicans in the setting of diaper dermatitis is unclear.
CLINICAL STUDIES
Study 1 was a double-blind, multicenter study in which VUSION Ointment was compared to the combination treatment of zinc oxide and white petrolatum (zinc oxide/white petrolatum) and enrolled 330 infants and toddlers with diaper dermatitis, 236 of whom were included in the modified intent-to-treat (MITT) population. The MITT population was defined as all subjects with confirmed Candida spp. who were dispensed study medication. In addition to diaper dermatitis at baseline, subjects were required to have candidiasis documented by KOH tests that demonstrated psuedohyphae and/or budding yeasts. Study medication was applied at every diaper change for 7 days.
The primary endpoint in Study 1 was “Overall Cure”, requiring that subjects be both clinically cured (total resolution of all signs and symptoms of infection) and
microbiologically eradicated. Primary efficacy was assessed 1 week following the
end of treatment, at Day 14.
Study 1 results are shown in Table 1.
|
a“Overall Cure” required that subjects be both clinically cured (total resolution of all signs and symptoms of infection) and microbiologically eradicated. bPrimary efficacy was assessed at Day 14, 1 week post-treatment. |
||
| Overall Curea |
VUSION Ointment n=112 |
Zinc Oxide/White Petrolatum n=124 |
|
Day 7 (End of Treatment) |
8 (7%) |
1 (0.8%) |
|
Day 14b |
26 (23%) |
12 (10%) |
Two additional studies provided supportive evidence of the clinical efficacy of VUSION Ointment for infants and toddlers with diaper dermatitis, some who cultured positive for C. albicans. However, in the two additional studies, the presence of candidal infection was not established in culture-positive subjects, as microscopic testing (e.g. KOH) was not done. Therefore, the positive culture results may have reflected colonization rather than infection.
INDICATIONS AND USAGE
VUSION Ointment is indicated for the adjunctive treatment of diaper dermatitis only when complicated by documented candidiasis (microscopic evidence of pseudohyphae and/or budding yeast), in immunocompetent pediatric patients 4 weeks and older. A positive fungal culture for Candida albicans is not adequate evidence of candidal infection since colonization with C. albicans can result in a positive culture. The presence of candidal infection should be established by microscopic evaluation prior to initiating treatment.
VUSION Ointment should be used as part of a treatment regimen that includes measures directed at the underlying diaper dermatitis, including gentle cleansing of the diaper area and frequent diaper changes.
VUSION Ointment should not be used as a substitute for frequent diaper changes. VUSION Ointment should not be used to prevent the occurrence of diaper dermatitis, since preventative use may result in the development of drug resistance.
CONTRAINDICATIONS
VUSION Ointment is contraindicated in those patients with a history of sensitivity reactions to any of its components. It should be discontinued if hypersensitivity is noted.
PRECAUTIONS
General: If irritation occurs or if the disease worsens, use of the medication should be discontinued, and the health care provider should be contacted. For external use only. VUSION Ointment is for topical use only, and not for ophthalmic, oral or intravaginal use.
The safety and efficacy of VUSION Ointment has not been demonstrated in immunocompromised patients, or in infants less than 4 weeks of age (premature or term).
The safety and efficacy of VUSION Ointment have not been evaluated in incontinent adult patients. VUSION Ointment should not be used to prevent the occurrence of diaper dermatitis, such as in an adult institutional setting, since preventative use may result in the development of drug resistance.
Information for Patients: Patients using VUSION Ointment should receive the following information and instructions: (See Patient Package Insert)
- VUSION Ointment is to be used only for diaper dermatitis that is complicated by documented candidiasis (i.e. documented by microscopic testing).
- VUSION Ointment should not be used as a substitute for frequent diaper changes.
- VUSION Ointment should not be used to prevent diaper dermatitis.
- VUSION Ointment should not be used long term.
- VUSION Ointment is to be used only as directed by the health care provider.
- VUSION Ointment is for external use only. It is not to be used orally, intravaginally, or for the eyes.
- Gently cleanse the diaper area with lukewarm water or a very mild soap and pat the area dry with a soft towel before applying VUSION Ointment.
- Gently apply VUSION Ointment to the diaper area with the fingertips after each diaper change. Do not rub VUSION Ointment into the skin as this may cause additional irritation.
- Thoroughly wash hands after applying VUSION Ointment.
- Treatment should be continued for 7 days, even if there is improvement. Do not use VUSION Ointment for longer than 7 days. If symptoms have not improved by day 7, see your health care provider.
- VUSION Ointment should not be used on children for whom it is not prescribed.
Drug Interactions: Drug-drug interaction studies were not conducted. Although women who take a warfarin anticoagulant and use a miconazole intravaginal cream or suppository may be at risk for developing an
increased prothrombin time, international normalized ratio (INR) and bleeding, the potential for this interaction to occur between warfarin and VUSION Ointment is unknown.
Carcinogenesis, Mutagenesis, Impairment of fertility: Studies to evaluate the carcinogenic potential of VUSION Ointment in animals have not been performed.
Miconazole nitrate was negative in a bacterial reverse mutation test, a chromosome aberration test in mice, and micronucleus assays in mice and rats.
Miconazole nitrate had no adverse effect on fertility in a study in rats at oral doses of up to 320 mg/kg/day, which is 89 times the maximum possible topical exposure of caregivers, assuming 100% absorption.
Pregnancy Category C:
There are no adequate and well-controlled studies of VUSION Ointment in pregnant women. Miconazole nitrate administration has been shown to result in prolonged gestation and decreased numbers of live young in rats and in increased number of resorptions and decreased number of live young in rabbits at oral doses of 100 mg/kg/day and 80 mg/kg/day, which are 28 and 45 times the maximum possible topical exposure of caregivers, respectively, assuming 100% absorption.
Pregnant women should exercise appropriate precautions when administering the product.
Nursing Mothers: Safety and efficacy of the product have not been established in nursing mothers. It is not known if the active components of VUSION Ointment may be present in milk. Nursing mothers should exercise appropriate precautions when administering the product.
Pediatric Use: Use: Efficacy was not demonstrated in infants less than 4 weeks of age. Use in infants below the age of 4 weeks is not recommended. Safety and efficacy have not been established in very-low-birth-weight infants.
VUSION Ointment should not be used to prevent diaper dermatitis.
The safety of VUSION Ointment when used for longer than 7 days is not known.
Geriatric Use: Clinical studies of VUSION Ointment did not include any subjects aged 65 and over. Safety and effectiveness in a geriatric population have not been evaluated.
ADVERSE REACTIONS
A total of 835 infants and young children were evaluated in the clinical development program. Of 418 subjects in the VUSION Ointment group, 58 (14%) reported one or more adverse events. Of 417 subjects in the zinc oxide/white petrolatum control group, 85 (20%) reported one or more adverse events. Adverse events that occurred at a rate of ≥ 1% for subjects who were treated with VUSION were approximately the same in type and frequency as for subjects who were treated with zinc oxide/white petrolatum ointment.
The potential for dermal toxicity of VUSION Ointment formulation was investigated in healthy adult volunteers in four topical safety studies. These studies were conducted to assess the potential for contact phototoxicity, photoallergy, sensitization, and cumulative irritation potential. Phototesting was conducted with UV-A only. Results indicated that VUSION Ointment did not induce a contact dermal phototoxic response, contact dermal photoallergic response, or contact dermal sensitization in adult subjects. In addition, VUSION Ointment did not show any evidence of cumulative irritation potential in adult subjects.
OVERDOSAGE
VUSION Ointment is intended for topical use only. Young children are at risk for
accidentally ingesting VUSION Ointment. A health care provider or poison control center should be contacted in the event of accidental ingestion.
DOSAGE AND ADMINISTRATION
Before applying VUSION Ointment, gently cleanse the skin with lukewarm water and pat dry with a soft towel. Avoid using any scented soaps, shampoos, or lotions on the diaper area.
VUSION Ointment should be applied to the affected area at each diaper change for 7 days. Treatment should be continued for the full 7 days, even if there is improvement. The safety of VUSION Ointment when used for longer than 7 days is not known.
Gently apply a thin layer of VUSION Ointment to the diaper area with the fingertips. VUSION Ointment should not be rubbed into the skin as this may cause additional irritation. Thoroughly wash hands after applying VUSION Ointment.
VUSION Ointment should be used for the adjunctive treatment of diaper dermatitis only when complicated by candidiasis, as documented by microscopic evidence of pseudohyphae and/or budding yeasts, in immunocompetent pediatric patients 4 weeks and older. VUSION Ointment should be used as part of a treatment regimen that includes measures directed at the underlying diaper dermatitis, including gentle cleansing of the diaper area and frequent diaper changes.
VUSION Ointment should not be used as a substitute for frequent diaper changes. VUSION Ointment should not be used to prevent the occurrence of diaper dermatitis, since or preventative use may result in the development of drug resistance.
HOW SUPPLIED
VUSION™ Ointment is a smooth, uniform, white ointment supplied in an aluminum tube, as follows:
5g sample (NDC13478-002-03)
30g (NDC 13478-002-01)
30g sample (NDC 13478-002-02)
Storage Conditions
Store at controlled room temperature between 20°C and 25°C (68°F and 77°F); with excursions permitted between 15°C and 30°C (59°F and 86°F).
Keep out of reach of children.
For additional information, please call toll free 1-866-440-5508.
Manufactured By:
DSM Pharmaceuticals, Inc.
Greenville, NC 27834
For:
Barrier Therapeutics, Inc.
600 College Road East
Princeton, NJ 08540
www.barriertherapeutics.com
VU-008 February, 2006
U.S. Patent No. 4,911,932
VUSION™
Patient Package Insert
(0.25% miconazole nitrate, 15% zinc oxide and 81.35% white petrolatum) Ointment
For Skin Use Only
Read the Patient Information that comes with VUSION Ointment before you use it on your child. This leaflet does not take the place of talking to your health care provider about your child’s medical condition or treatment. If you have any questions or if you are not sure about any of the information on VUSION Ointment, ask your health care provider, or pharmacist.
What is VUSION Ointment?
VUSION Ointment is a prescription skin medicine used to treat diaper rash that also has a yeast infection in children who have a normal immune system. VUSION Ointment contains medicines that will help treat the yeast infection and the diaper rash, but you must also change your child’s diapers very often so that your child is not wearing a wet or soiled diaper. Even if you use VUSION
Ointment, diaper rash will not go away if you do not keep your child’s diaper area clean and dry. You should use water or a very mild cleanser to clean your child’s diaper area. VUSION Ointment is not used to prevent diaper rash or to be used for more than 7 days.
Who should not use VUSION Ointment?
VUSION Ointment is not for treatment of all cases of diaper rash. VUSION Ointment is only for diaper rash that also has a yeast infection. Most cases of diaper rash do not need the yeast medicine that is in VUSION Ointment because most cases of diaper rash do not also have a yeast infection.
Your health care provider will need to do a special test to tell if your child’s diaper rash also has a yeast infection. Do not use VUSION Ointment on your child’s diaper rash unless your health care provider tells you that there is also a yeast infection.
Do not use VUSION Ointment on any other children.
Do not use VUSION Ointment on your child’s diaper rash if they are allergic to anything in it. See the end of this leaflet for a list of ingredients in VUSION Ointment.
How should I use VUSION Ointment on my child?
VUSION Ointment is applied to the skin on your child’s diaper area at each diaper change for 7 days.
Apply VUSION Ointment for the full 7 days even if the diaper rash starts to go away. Call your child’s health care provider if the diaper rash gets worse or does not go away with 7 days of treatment with VUSION Ointment. VUSION Ointment should not be used for more than 7 days.
To apply VUSION Ointment:
- Gently, clean the skin on your child’s diaper area with warm (not hot) water. You may also use a very mild soap. Pat the area dry with a soft towel.
- Use your fingertips and gently apply a thin layer of VUSION Ointment to your child’s diaper area at each diaper change. Do not rub VUSION Ointment into your child’s skin. Rubbing the skin can cause more irritation.
- Wash your hands after applying VUSION Ointment on your child.
VUSION Ointment is for skin use only.
Call your child’s health care provider or poison control center right away if any VUSION Ointment is swallowed. Call your child’s health care provider if VUSION Ointment gets in the eye.
What other steps will help diaper rash go away?
- Check your child’s diaper often. Change the diaper at the first sign of wetness.
- Clean your child’s diaper area after each diaper change. Gently wipe the diaper area from the front to back using warm (not hot) water. You may also use a mild soap. Rinse the diaper area well. Pat dry with a soft towel.
- Keep the diaper area open to air when possible.
- Even if you use VUSION Ointment, diaper rash will not go away if you do not keep your child’s diaper area clean and dry.
What are the possible side effects of VUSION Ointment?
Ointment may cause irritation, but this is not common. You should call your child’s health care provider if irritation appears or if the diaper rash gets worse.
How should I store VUSION Ointment?
- Store VUSION Ointment at room temperature between 59° to 86°F (15° to 30°C).
- Keep VUSION Ointment out of the reach of children.
General information about VUSION Ointment
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
Do not use VUSION Ointment for a condition for which it was not prescribed. Do not give VUSION Ointment to other children, even if they have the same symptoms your child has. It may harm them.
This leaflet summarizes the most important information about VUSION Ointment. If you would like more information, talk to your child’s health care provider. You can ask your child’s health care provider or pharmacist for information about VUSION Ointment that is written for healthcare professionals.
You can also call Barrier Therapeutics, Inc Customer Information Center toll free at 1-866-440-5508.
What are the ingredients in VUSION Ointment?
Active Ingredients: miconazole nitrate, zinc oxide and white petrolatum
Inactive Ingredients: trihydroxystearin, butylated hydroxyltoluene (BHT) and Chemoderm® 1001/B fragrance
Marketed by:
Barrier Therapeutics, Inc.
Princeton, NJ 08540-6697 USA
www.barriertherapeutics.com
Manufactured by:
DSM Pharmaceuticals, Inc.
Greenville, NC 27834 USA
February, 2006
VU-009
U.S. Patent No. 4,911,932
| Vusion (miconazole nitrate, zinc oxide and white petrolatum) | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Revised: 04/2007Barrier Therapeutics, Inc.