ketorolac tromethamine (Ketorolac Tromethamine) tablet, film coated
[Mylan Pharmaceuticals Inc.]
10 mg
Rx only
WARNING
Ketorolac tromethamine, a nonsteroidal anti-inflammatory drug (NSAID), is indicated for the short-term (up to 5 days) management of moderately severe, acute pain, that requires analgesia at the opioid level. It is NOT indicated for minor or chronic painful conditions. Ketorolac tromethamine is a potent NSAID analgesic, and its administration carries many risks. The resulting NSAID-related adverse events can be serious in certain patients for whom ketorolac tromethamine is indicated, especially when the drug is used inappropriately. Increasing the dose of ketorolac tromethamine beyond the label recommendations will not provide better efficacy but will result in increasing the risk of developing serious adverse events.
GASTROINTESTINAL EFFECTS
- Ketorolac tromethamine can cause peptic ulcers, gastrointestinal bleeding, and/or perforation. Therefore, ketorolac tromethamine is CONTRAINDICATED in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding.
RENAL EFFECTS
- Ketorolac tromethamine is CONTRAINDICATED in patients with advanced renal impairment and in patients at risk for renal failure due to volume depletion (see WARNINGS).
RISK OF BLEEDING
- Ketorolac tromethamine inhibits platelet function and is, therefore, CONTRAINDICATED in patients with suspected or confirmed cerebrovascular bleeding, patients with hemorrhagic diathesis, incomplete hemostasis, and those at high risk of bleeding (see WARNINGS and PRECAUTIONS).
- Ketorolac tromethamine is CONTRAINDICATED as prophylactic analgesic before any major surgery, and is CONTRAINDICATED intra-operatively when hemostasis is critical because of the increased risk of bleeding.
HYPERSENSITIVITY
- Hypersensitivity reactions, ranging from bronchospasm to anaphylactic shock, have occurred and appropriate counteractive measures must be available when administering the first dose of ketorolac tromethamine-IV/IM (see CONTRAINDICATIONS and WARNINGS). Ketorolac tromethamine is CONTRAINDICATED in patients with previously demonstrated hypersensitivity to ketorolac tromethamine, or allergic manifestations to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs).
LABOR, DELIVERY, AND NURSING
- The use of ketorolac tromethamine in labor and delivery is CONTRAINDICATED because it may adversely effect fetal circulation and the uterus.
- The use of ketorolac tromethamine is CONTRAINDICATED in nursing mothers because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates.
CONCOMITANT USE WITH NSAIDs
- Ketorolac tromethamine is CONTRAINDICATED in patients currently receiving ASA or NSAIDs because of the cumulative risk of inducing serious NSAID-related side effects.
DOSAGE AND ADMINISTRATION
KETOROLAC TROMETHAMINE TABLETS
- Ketorolac tromethamine tablets are indicated only as continuation therapy to ketorolac tromethamine-IV/IM, and the combined duration of use of ketorolac tromethamine-IV/IM and ketorolac tromethamine tablets is not to exceed 5 (five) days, because of the increased risk of serious adverse events.
- The recommended total daily dose of ketorolac tromethamine tablets (maximum 40 mg) is significantly lower than for ketorolac tromethamine-IV/IM (maximum 120 mg) (see DOSAGE and ADMINISTRATION and Transition from ketorolac tromethamine-IV/IM to ketorolac tromethamine tablets).
SPECIAL POPULATIONS
- Dosage should be adjusted for patients 65 years or older, for patients under 50 kg (110 lbs) of body weight (see DOSAGE and ADMINISTRATION), and for patients with moderately elevated serum creatinine (see WARNINGS).
DESCRIPTION
Ketorolac tromethamine is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs). The chemical name for ketorolac tromethamine is (±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol, and the structural formula is:
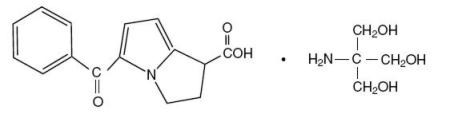
C15H13NO3 • C4H11NO3
Ketorolac tromethamine is a racemic mixture of [-]S and [+]R ketorolac tromethamine. Ketorolac tromethamine may exist in three crystal forms. All forms are equally soluble in water. Ketorolac tromethamine has a pKa of 3.5 and an n-octanol/water partition coefficient of 0.26. The molecular weight of ketorolac tromethamine is 376.41.
Each tablet for oral administration contains 10 mg ketorolac tromethamine. In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, croscarmellose sodium, glyceryl triacetate, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, sodium lauryl sulfate and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug (NSAID). Ketorolac tromethamine inhibits synthesis of prostaglandins and may be considered a peripherally acting analgesic. The biological activity of ketorolac tromethamine is associated with the S-form. Ketorolac tromethamine possesses no sedative or anxiolytic properties.
Pain relief was statistically different after ketorolac tromethamine dosing from that of placebo at 1/2 hour (the first time point at which it was measured) following the largest recommended doses of ketorolac tromethamine, and by 1 hour following the smallest recommended doses. The peak analgesic effect occurred within 2 to 3 hours and was not statistically significantly different over the recommended dosage range of ketorolac tromethamine. The greatest difference between large and small doses of ketorolac tromethamine by either route was in the duration of analgesia.
Pharmacokinetics
Ketorolac tromethamine is a racemic mixture of [-]S- and [+]R-enantiomeric forms, with the S-form having analgesic activity.
Comparison of IV, IM, and Oral Pharmacokinetics
The pharmacokinetics of ketorolac tromethamine, following IV, IM, and oral doses of ketorolac tromethamine, are compared in Table 1. The extent of bioavailability following administration of the oral and IM forms of ketorolac tromethamine was equal to that following an IV bolus.
Linear Kinetics
Following administration of single oral, IM, or IV doses of ketorolac tromethamine, in the recommended dosage ranges, the clearance of the racemate does not change. This implies that the pharmacokinetics of ketorolac tromethamine in humans, following single or multiple IM, IV, or recommended oral doses of ketorolac tromethamine, are linear. At the higher recommended doses, there is a proportional increase in the concentrations of free and bound racemate.
Binding and Distribution
The ketorolac tromethamine racemate has been shown to be highly protein-bound (99%). Nevertheless, even plasma concentrations as high as 10 mcg/mL will only occupy approximately 5% of the albumin binding sites. Thus, the unbound fraction for each enantiomer will be constant over the therapeutic range. A decrease in serum albumin, however, will result in increased free drug concentrations.
The mean apparent volume (Vβ) of ketorolac tromethamine following complete distribution was approximately 13 liters. This parameter was determined from single dose data.
Metabolism
Ketorolac tromethamine is largely metabolized in the liver. The metabolic products are hydroxylated and conjugated forms of the parent drug. The products of metabolism, and some unchanged drug, are excreted in the urine.
Clearance and Excretion
A single-dose study with 10 mg ketorolac tromethamine (n = 9) demonstrated that the S-enantiomer is cleared approximately two times faster than the R-enantiomer, and that the clearance was independent of the route of administration. This means that the ratio of S/R plasma concentrations decreases with time after each dose. There is little or no inversion of the R- to S- form in humans. The clearance of the racemate in normal subjects, elderly individuals, and in hepatically and renally impaired patients, is outlined in Table 2.
The half-life of the ketorolac tromethamine S-enantiomer was approximately 2.5 hours (SD ± 0.4) compared with 5 hours (SD ± 1.7) for the R-enantiomer. In other studies, the half-life for the racemate has been reported to lie within the range of 5 to 6 hours.
Accumulation
Ketorolac tromethamine administered as an IV bolus, every 6 hours, for 5 days, to healthy subjects (n = 13), showed no significant difference in Cmax on Day 1 and Day 5. Trough levels averaged 0.29 mcg/mL (SD ± 0.13) on Day 1 and 0.55 mcg/mL (SD ± 0.23) on Day 6. Steady-state was approached after the fourth dose.
Accumulation of ketorolac tromethamine has not been studied in special populations (elderly patients, renal failure patients, or hepatic disease patients).
Effect of Food
Oral administration of ketorolac tromethamine after a high fat meal resulted in decreased peak and delayed time-to-peak concentrations of ketorolac tromethamine by about 1 hour. Antacids did not affect the extent of absorption.
Kinetics in Special Populations
Elderly Patients
Based on single-dose data only, the half-life of the ketorolac tromethamine racemate increased from 5 to 7 hours in the elderly (65–78 years) compared with young healthy volunteers (24 to 35 years) (see Table 2). There was little difference in the Cmax for the two groups (elderly, 2.52 mcg/mL ± 0.77; young, 2.99 mcg/mL ± 1.03) (see PRECAUTIONS: Use in the Elderly).
Renally Impaired Patients
Based on single-dose data only, the mean half-life of ketorolac tromethamine in renally impaired patients is between 6 and 19 hours, and is dependent on the extent of the impairment. There is poor correlation between creatinine clearance and total ketorolac tromethamine clearance in the elderly and populations with renal impairment (r = 0.5).
In patients with renal disease, the AUC∞ of each enantiomer increased by approximately 100% compared with healthy volunteers. The volume of distribution doubles for the S-enantiomer and increases by 1/5th for the R-enantiomer. The increase in volume of distribution of ketorolac tromethamine implies an increase in unbound fraction.
The AUC∞-ratio of the ketorolac tromethamine enantiomers in healthy subjects and patients remained similar, indicating there was no selective excretion of either enantiomer in patients compared to healthy subjects (see WARNINGS: Renal Effects).
Hepatic Effects
There was no significant difference in estimates of half-life, AUC∞, Cmax, in 7 patients with liver disease compared to healthy volunteers (see PRECAUTIONS: General: Hepatic Effects).
| Pharmacokinetic Parameters (units) | Oral* | Intramuscular† | Intravenous Bolus‡ | |||
|---|---|---|---|---|---|---|
| 10mg | 15mg | 30mg | 60mg | 15mg | 30mg | |
| % Dose metabolized = < 50 % Dose excreted in urine = 91 % Dose excreted in feces = 6 % Plasma protein binding = 99 |
||||||
|
||||||
| Bioavailability (extent) | 100% | |||||
| Tmax§ (min) | 44 ± 34 | 33 ± 21¶ | 44 ± 29 | 33 ± 21¶ | 1.1 ± 0.7¶ | 2.9 ± 1.8 |
| Cmax# (mcg/mL) [single dose] | 0.87 ± 0.22 | 1.14 ± 0.32¶ | 2.42 ± 0.68 | 4.55 ± 1.27¶ | 2.47 ± 0.51¶ | 4.65 ± 0.96 |
| Cmax (mcg/mL) [steady state q.i.d.] | 1.05 ± 0.26¶ | 1.56 ± 0.44¶ | 3.11 ± 0.87¶ | N/AÞ | 3.09 ± 1.17¶ | 6.85 ± 2.61 |
| Cminß (mcg/mL) [steady state q.i.d.] | 0.29 ± 0.07¶ | 0.47 ± 0.13¶ | 0.93 ± 0.26¶ | N/A | 0.61± 0.21¶ | 1.04 ± 0.35 |
| Caveà (mcg/mL) [steady state q.i.d.] | 0.59 ± 0.20¶ | 0.94 ± 0.29¶ | 1.88 ± 0.59¶ | N/A | 1.09 ± 0.30¶ | 2.17 ± 0.59 |
| Vβè (L/kg) | 0.175 ± 0.039 | 0.210 ± 0.044 | ||||
| Types of Subjects | Total Clearance [in L/h/kg]‡ | Terminal Half-Life [in hours] |
||
|---|---|---|---|---|
| IM Mean(range) | ORAL Mean(range) | IM Mean(range) | ORAL Mean(range) |
|
| Normal Subjects | ||||
| IM (n=54) mean age=32, range=18–60 | 0.023 | 0.025 | 5.3 | 5.3 |
| Oral (n=77) mean age=32, range=20–60 | (0.010–0.046) | (0.013–0.050) | (3.5–9.2) | (2.4–9.0) |
| Healthy Elderly Subjects | 0.019 | 0.024 | 7.0 | 6.1 |
| IM (n=13), Oral (n=12) | (0.013–0.034) | (0.018–0.034) | (4.7–8.6) | (4.3–7.6) |
| mean age =72, range =65–78 | ||||
| Patients with Hepatic Dysfunction | 0.029 | 0.033 | 5.4 | 4.5 |
| IM and Oral (n=7) | (0.013–0.066) | (0.019–0.051) | (2.2–6.9) | (1.6–7.6) |
| mean age=51, range=43–64 | ||||
| Patients with Renal Impairment IM (n=25), Oral (n=9) | 0.015 | 0.016 | 10.3 | 10.8 |
| serum creatinine=1.9–5.0 mg/dL | (0.005–0.043) | (0.007–0.052) | (5.9–19.2) | (3.4–18.9) |
| mean age (IM)=54, range 35–71 mean age (oral)=57, range=39–70 | ||||
| Renal Dialysis Patients | 0.016 | — | 13.6 | — |
| IM and Oral (n=9), | (0.003–0.036) | (8.0–39.1) | ||
| mean age=40, range=27–63 | ||||
| IV Administration: | In normal subjects (n = 37), the total clearance of 30 mg IV administered ketorolac tromethamine was 0.030 (0.017–0.051) L/h/kg. The terminal half-life was 5.6 (4.0–7.9) hours. |
Clinical Studies
The analgesic efficacy of intramuscularly, intravenously and orally administered ketorolac tromethamine was investigated in two postoperative pain models: general surgery (orthopedic, gynecologic and abdominal) and oral surgery (removal of impacted third molars). The studies were double-blind, single- and multiple-dose, parallel trial designs, in patients with moderate to severe pain at baseline. Ketorolac tromethamine-IV/IM was compared as follows: IM to meperidine or morphine administered intramuscularly, and IV to morphine administered either directly IV or through a PCA (Patient-Controlled Analgesia) pump.
Short-Term Use (up to 5 days) Studies
In the comparisons of intramuscular administration during the first hour, the onset of analgesic action was similar for ketorolac tromethamine and the narcotics, but the duration of analgesia was longer with ketorolac tromethamine than with the opioid comparators meperidine or morphine.
In a multi-dose, postoperative (general surgery) double-blind trial of ketorolac tromethamine-IM 30 mg versus morphine 6 and 12 mg IM, each drug given on an "as needed" basis for up to 5 days, the overall analgesic effect of ketorolac tromethamine-IM 30 mg was between that of morphine 6 and 12 mg. The majority of patients treated with either ketorolac tromethamine or morphine were dosed for up to 3 days; a small percentage of patients received 5 days of dosing.
In clinical settings where perioperative morphine was allowed, ketorolac tromethamine-IV 30 mg, given once or twice as needed, provided analgesia comparable to morphine 4 mg IV once or twice as needed.
There was relatively limited experience with 5 consecutive days of ketorolac tromethamine-IV use in controlled clinical trials, as most patients were given the drug for 3 days or less. The adverse events seen with IV-administered ketorolac tromethamine were similar to those observed with IM-administered ketorolac tromethamine, as would be expected based on the similar pharmacokinetics and bioequivalence (AUC, clearance, plasma half-life) of IV and IM routes of ketorolac tromethamine administration.
Clinical Studies with Concomitant Use of Opioids
Clinical studies in postoperative pain management have demonstrated that ketorolac tromethamine-IV/IM, when used in combination with opioids, significantly reduced opioid consumption. This combination may be useful in the subpopulation of patients especially prone to opioid-related complications. Ketorolac tromethamine and narcotics should not be administered in the same syringe.
In a postoperative study, where all patients received morphine by a PCA device, patients treated with ketorolac tromethamine-IV as fixed intermittent boluses (e.g., 30 mg initial dose followed by 15 mg q3h), required significantly less morphine (26%) than the placebo group. Analgesia was significantly superior, at various postdosing pain assessment times, in the patients receiving ketorolac tromethamine-IV plus PCA morphine as compared to patients receiving PCA-administered morphine alone.
Postmarketing Surveillance Study
A large postmarketing observational, non-randomized study, involving approximately 10,000 patients receiving ketorolac tromethamine, demonstrated that the risk of clinically serious gastrointestinal (G.I.) bleeding was dose-dependent (see Table 3A and 3B). This was particularly true in elderly patients who received an average daily dose greater than 60 mg/day of ketorolac tromethamine (Table 3A).
| A. Patients without History of PUB | ||||
|---|---|---|---|---|
| Age of Patients | Total Daily Dose of Ketorolac Tromethamine IV/IM | |||
| ≤ 60 mg | > 60 to 90 mg | > 90 to 120 mg | > 120 mg | |
| < 65 years of age | 0.4% | 0.4% | 0.9% | 4.6% |
| ≥ 65 years of age | 1.2% | 2.8% | 2.2% | 7.7% |
| B. Patients with History of PUB | ||||
|---|---|---|---|---|
| Age of Patients | Total Daily Dose of Ketorolac Tromethamine IV/IM | |||
| ≤ 60 mg | > 60 to 90 mg | > 90 to 120 mg | > 120 mg | |
| < 65 years of age | 2.1% | 4.6% | 7.8% | 15.4% |
| ≥ 65 years of age | 4.7% | 3.7% | 2.8% | 25.0% |
INDICATIONS AND USAGE
Ketorolac tromethamine is indicated for the short-term (≤ 5 days) management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a postoperative setting. Therapy should always be initiated with ketorolac tromethamine-IV/IM, and ketorolac tromethamine tablets are to be used only as continuation treatment, if necessary. Combined use of ketorolac tromethamine-IV/IM and ketorolac tromethamine tablets is not to exceed 5 days of use because of the potential of increasing the frequency and severity of adverse reactions associated with the recommended doses (see WARNINGS, PRECAUTIONS, DOSAGE AND ADMINISTRATION, and ADVERSE REACTIONS). Patients should be switched to alternative analgesics as soon as possible, but ketorolac tromethamine therapy is not to exceed 5 days.
CONTRAINDICATIONS
(see also Boxed WARNING)
Ketorolac tromethamine is CONTRAINDICATED in patients with active peptic ulcer disease, in patients with recent gastrointestinal bleeding or perforation, and in patients with a history of peptic ulcer disease or gastrointestinal bleeding.
Ketorolac tromethamine is CONTRAINDICATED in patients with advanced renal impairment, or in patients at risk for renal failure due to volume depletion (see WARNINGS for correction of volume depletion).
Ketorolac tromethamine is CONTRAINDICATED in labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine musculature, thus increasing the risk of uterine hemorrhage.
The use of ketorolac tromethamine is CONTRAINDICATED in nursing mothers because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates.
Ketorolac tromethamine is CONTRAINDICATED in patients with previously demonstrated hypersensitivity to ketorolac tromethamine, or allergic manifestations to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs).
Ketorolac tromethamine is CONTRAINDICATED as prophylactic analgesic before any major surgery, and is CONTRAINDICATED intra-operatively when hemostasis is critical because of the increased risk of bleeding.
Ketorolac tromethamine inhibits platelet function and is, therefore, CONTRAINDICATED in patients with suspected or confirmed cerebrovascular bleeding, hemorrhagic diathesis, incomplete homeostasis, and those at high risk of bleeding (see WARNINGS and PRECAUTIONS).
Ketorolac tromethamine is CONTRAINDICATED in patients currently receiving ASA or NSAIDs because of the cumulative risks of inducing serious NSAID related adverse events.
The concomitant use of ketorolac tromethamine and probenecid is CONTRAINDICATED.
WARNINGS
(see also Boxed WARNING)
The combined use of ketorolac tromethamine-IV/IM and ketorolac tromethamine tablets is not to exceed 5 days. The most serious risks associated with ketorolac tromethamine are:
Gastrointestinal Ulcerations, Bleeding and Perforation
Ketorolac tromethamine is contraindicated in patients with previously documented peptic ulcers and/or G.I. bleeding. Serious gastrointestinal toxicity, such as bleeding, ulceration, and perforation, can occur at any time, with or without warning symptoms, in patients treated with ketorolac tromethamine. Studies to date with NSAIDs have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals, and most spontaneous reports of fatal GI events are in this population. Postmarketing experience with parenterally administered ketorolac tromethamine suggests that there may be a greater risk of gastrointestinal ulcerations, bleeding and perforation in the elderly.
The incidence and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with, ketorolac tromethamine. In a non-randomized, in-hospital postmarketing surveillance study, comparing parenteral ketorolac tromethamine to parenteral opioids, higher rates of clinically serious G.I. bleeding were seen in patients < 65 years of age who received an average total daily dose of more than 90 mg of ketorolac tromethamine-IV/IM per day (see CLINICAL PHARMACOLOGY: Clinical Studies: Postmarketing Surveillance Study).
The same study showed that elderly (≥ 65 years of age), and debilitated patients are more susceptible to gastrointestinal complications. A history of peptic ulcer disease was revealed as another risk factor that increases the possibility of developing serious gastrointestinal complications during ketorolac tromethamine therapy (see Tables 3A and B).
Impaired Renal Function
Ketorolac tromethamine should be used with caution in patients with impaired renal function, or a history of kidney disease because it is a potent inhibitor of prostaglandin synthesis. Renal toxicity with ketorolac tromethamine has been seen in patients with conditions leading to a reduction in blood volume and/or renal blood flow, where renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients, administration of ketorolac tromethamine may cause a dose-dependent reduction in renal prostaglandin formation and may precipitate acute renal failure. Patients at greatest risk of this reaction are those with impaired renal function, dehydration, heart failure, liver dysfunction, those taking diuretics and the elderly. Discontinuation of ketorolac tromethamine therapy is usually followed by recovery to the pretreatment state.
Renal Effects
Ketorolac tromethamine and its metabolites are eliminated primarily by the kidneys, which, in patients with reduced creatinine clearance, will result in diminished clearance of the drug (see CLINICAL PHARMACOLOGY). Therefore, ketorolac tromethamine should be used with caution in patients with impaired renal function (see DOSAGE AND ADMINISTRATION) and such patients should be followed closely. With the use of ketorolac tromethamine, there have been reports of acute renal failure, nephritis, and nephrotic syndrome.
Because patients with underlying renal insufficiency are at increased risk of developing acute renal failure, the risks and benefits should be assessed prior to giving ketorolac tromethamine to these patients. Ketorolac tromethamine is CONTRAINDICATED IN PATIENTS WITH SERUM CREATININE CONCENTRATIONS INDICATING ADVANCED RENAL IMPAIRMENT (see CONTRAINDICATIONS).
Hypovolemia should be corrected before treatment with ketorolac tromethamine is initiated.
Fluid Retention and Edema
Fluid retention, edema, retention of NaCl, oliguria, elevations of serum urea nitrogen and creatinine have been reported in clinical trials with ketorolac tromethamine. Therefore, ketorolac tromethamine should be used only very cautiously in patients with cardiac decompensation, hypertension, or similar conditions.
Hemorrhage
Because prostaglandins play an important role in hemostasis, and NSAIDs affect platelet aggregation as well, use of ketorolac tromethamine in patients who have coagulation disorders should be undertaken very cautiously, and those patients should be carefully monitored. Patients on therapeutic doses of anticoagulants (e.g., heparin or dicumarol derivatives) have an increased risk of bleeding complications if given ketorolac tromethamine concurrently; therefore, physicians should administer such concomitant therapy only extremely cautiously. The concurrent use of ketorolac tromethamine and prophylactic low-dose heparin (2500 to 5000 units q12h), warfarin and dextrans have not been studied extensively, but may also be associated with an increased risk of bleeding. Until data from such studies are available, physicians should carefully weigh the benefits against the risks, and use such concomitant therapy in these patients only extremely cautiously. In patients who receive anticoagulants for any reason, there is an increased risk of intramuscular hematoma formation from administered ketorolac tromethamine-IM (see PRECAUTIONS: Drug Interactions). Patients receiving therapy that affects hemostasis should be monitored closely.
In postmarketing experience, postoperative hematomas and other signs of wound bleeding have been reported in association with the perioperative use of ketorolac tromethamine-IV/IM. Therefore, perioperative use of ketorolac tromethamine should be avoided and postoperative use be undertaken with caution when hemostasis is critical (see WARNINGS and PRECAUTIONS).
Anaphylactoid Reactions
Anaphylactoid reactions may occur in patients without a known previous exposure or hypersensitivity to aspirin, ketorolac tromethamine, or other NSAIDs, or in individuals with a history of angioedema, bronchospastic reactivity (e.g., asthma), and nasal polyps. Anaphylactoid reactions, like anaphylaxis, may have a fatal outcome.
PRECAUTIONS
General
Hepatic Effects
Ketorolac tromethamine should be used with caution in patients with impaired hepatic function, or a history of liver disease. Treatment with ketorolac tromethamine may cause elevations of liver enzymes, and in patients with pre-existing liver dysfunction it may lead to the development of a more severe hepatic reaction. The administration of ketorolac tromethamine should be discontinued in patients in whom an abnormal liver test has occurred as a result of ketorolac tromethamine therapy.
Hematologic Effects
Ketorolac tromethamine inhibits platelet aggregation and may prolong bleeding time; therefore, it is contraindicated as a preoperative medication and caution should be used when hemostasis is critical. Unlike aspirin, the inhibition of platelet function by ketorolac tromethamine disappears within 24 to 48 hours after the drug is discontinued. Ketorolac tromethamine does not appear to affect platelet count, prothrombin time (PT) or partial thromboplastin time (PTT). In controlled clinical studies, where ketorolac tromethamine was administered intramuscularly or intravenously postoperatively, the incidence of clinically significant postoperative bleeding was 0.4% for ketorolac tromethamine compared to 0.2% in the control groups receiving narcotic analgesics.
Information for Patients
Ketorolac tromethamine is a potent NSAID and may cause serious side effects such as gastrointestinal bleeding or kidney failure, which may result in hospitalization and even fatal outcome.
Physicians, when prescribing ketorolac tromethamine, should inform their patients of the potential risks of ketorolac tromethamine treatment (see Boxed WARNING, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS). Advise patients not to give ketorolac tromethamine tablets to other family members and to discard any unused drug. Remember that the total duration of ketorolac tromethamine therapy is not to exceed 5 (five) days.
Drug Interactions
Ketorolac is highly bound to human plasma protein (mean 99.2%).
The in vitro binding of warfarin to plasma proteins is only slightly reduced by ketorolac tromethamine (99.5% control vs 99.3%) when ketorolac plasma concentrations reach 5 to 10 mcg/mL. Ketorolac does not alter digoxin protein binding. In vitro studies indicate that, at therapeutic concentrations of salicylate (300 mcg/mL), the binding of ketorolac was reduced from approximately 99.2% to 97.5%, representing a potential two-fold increase in unbound ketorolac plasma levels. Therapeutic concentrations of digoxin, warfarin, ibuprofen, naproxen, piroxicam, acetaminophen, phenytoin, and tolbutamide did not alter ketorolac tromethamine protein binding.
In a study involving 12 volunteers, ketorolac tromethamine tablets were co-administered with a single-dose of 25 mg warfarin, causing no significant changes in pharmacokinetics or pharmacodynamics of warfarin. In another study, ketorolac tromethamine-IV/IM was given with two doses of 5000 U of heparin to 11 healthy volunteers, resulting in a mean template bleeding time of 6.4 minutes (3.2 to 11.4 min) compared to a mean of 6.0 minutes (3.4 to 7.5 min) for heparin alone and 5.1 minutes (3.5 to 8.5 min) for placebo. Although these results do not indicate a significant interaction between ketorolac tromethamine and warfarin or heparin, the administration of ketorolac tromethamine to patients taking anticoagulants should be done extremely cautiously and patients should be closely monitored (see WARNINGS and PRECAUTIONS).
Ketorolac tromethamine-IV/IM reduced the diuretic response to furosemide in normovolemic healthy subjects by approximately 20% (mean sodium and urinary output decreased 17%).
Concomitant administration of ketorolac tromethamine tablets and probenecid resulted in decreased clearance of ketorolac and significant increases in ketorolac plasma levels (total AUC increased approximately 3-fold from 5.4 to 17.8 mcg/h/mL) and terminal half-life increased approximately 2-fold from 6.6 to 15.1 hours. Therefore, concomitant use of ketorolac tromethamine and probenecid is contraindicated.
Inhibition of renal lithium clearance, leading to an increase in plasma lithium concentration, has been reported with some prostaglandin synthesis inhibiting drugs. The effect of ketorolac tromethamine on plasma lithium has not been studied, but cases of increased lithium plasma levels during ketorolac tromethamine therapy have been reported.
Concomitant administration of methotrexate and some NSAIDs has been reported to reduce the clearance of methotrexate, enhancing the toxicity of methotrexate. The effect of ketorolac tromethamine on methotrexate clearance has not been studied.
In postmarketing experience, there have been reports of a possible interaction between ketorolac tromethamine-IV/IM and non-depolarizing muscle relaxants that resulted in apnea. The concurrent use of ketorolac tromethamine with muscle relaxants has not been formally studied.
Concomitant use of ACE inhibitors may increase the risk of renal impairment, particularly in volume depleted patients.
Sporadic cases of seizures have been reported during concomitant use of ketorolac tromethamine and antiepileptic drugs (phenytoin sodium, carbamazepine).
Hallucinations have been reported when ketorolac tromethamine was used in patients taking psychoactive drugs (fluoxetine HCl, thiothixene, alprazolam).
There is no evidence, in animal or human studies, that ketorolac tromethamine induces or inhibits hepatic enzymes capable of metabolizing itself or other drugs.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
An 18-month study in mice with oral doses of ketorolac tromethamine at 2 mg/kg/day (0.9 times the human systemic exposure at the recommended IM or IV dose of 30 mg q.i.d., based on area-under-the-plasma-concentration curve [AUC]), and a 24-month study in rats at 5 mg/kg/day (0.5 times the human AUC), showed no evidence of tumorigenicity.
Ketorolac tromethamine was not mutagenic in the Ames test, unscheduled DNA synthesis and repair, and in forward mutation assays. Ketorolac tromethamine did not cause chromosome breakage in the in vivo mouse micronucleus assay. At 1590 mcg/mL and at higher concentrations, ketorolac tromethamine increased the incidence of chromosomal aberrations in Chinese hamster ovarian cells.
Impairment of fertility did not occur in male or female rats at oral doses of 9 mg/kg (0.9 times the human AUC) and 16 mg/kg (1.6 times the human AUC) of ketorolac tromethamine, respectively.
Pregnancy
Pregnancy Category C
Reproduction studies have been performed during organogenesis, using daily oral doses of ketorolac tromethamine at 3.6 mg/kg (0.37 times the human AUC) in rabbits and at 10 mg/kg (1.0 times the human AUC) in rats. Results of these studies did not reveal evidence of teratogenicity to the fetus. Oral doses of ketorolac tromethamine at 1.5 mg/kg (0.14 times the human AUC), administered after gestation day 17, caused dystocia and higher pup mortality in rats. There are no adequate and well-controlled studies of ketorolac tromethamine in pregnant women. Ketorolac tromethamine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery:
The use of ketorolac tromethamine is contraindicated in labor and delivery because, through its prostaglandin synthesis inhibitory effect, it may adversely affect fetal circulation and inhibit uterine musculature, thus increasing the risk of uterine hemorrhage (see CONTRAINDICATIONS).
Lactation and Nursing
After a single administration of 10 mg of oral ketorolac tromethamine to humans, the maximum milk concentration observed was 7.3 ng/mL and the maximum milk-to-plasma ratio was 0.037. After one day of dosing (q.i.d.), the maximum milk concentration was 7.9 ng/mL and the maximum milk-to-plasma ratio was 0.025. Because of the possible adverse effects of prostaglandin-inhibiting drugs on neonates, use in nursing mothers is CONTRAINDICATED.
Pediatric Use
Safety and efficacy in pediatric patients (less than 16 years of age) have not been established. Therefore, use of ketorolac tromethamine in pediatric patients is not recommended.
Use in the Elderly (≥ 65 years of age)
Because ketorolac tromethamine may be cleared more slowly by the elderly (see CLINICAL PHARMACOLOGY) who are also more sensitive to the adverse effects of NSAIDs (see WARNINGS: Renal Effects), extra caution and reduced dosages (see DOSAGE AND ADMINISTRATION) must be used when treating the elderly with ketorolac tromethamine. The incidences and severity of gastrointestinal complications increases with increasing dose of, and duration of treatment with, ketorolac tromethamine.
ADVERSE REACTIONS
Adverse reaction rates increase with higher doses of ketorolac tromethamine. Practitioners should be alert for the severe complications of treatment with ketorolac tromethamine, such as G.I. ulceration, bleeding and perforation, postoperative bleeding, acute renal failure, anaphylactic and anaphylactoid reactions, and liver failure (see Boxed WARNING, WARNINGS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION). These NSAID-related complications can be serious in certain patients for whom ketorolac tromethamine is indicated, especially when the drug is used inappropriately.
The adverse reactions listed below were reported in clinical trials as probably related to ketorolac tromethamine.
Incidence Greater Than 1%
[Percentage of incidence in parentheses for those events reported in 3% or more patients]:
Body as a Whole: edema (4%).
Cardiovascular: hypertension.
Dermatologic: pruritus, rash.
Gastrointestinal: nausea (12%), dyspepsia (12%), gastrointestinal pain (13%), diarrhea (7%), constipation, flatulence, gastrointestinal fullness, vomiting, stomatitis.
Hemic and Lymphatic: purpura.
Nervous System: headache (17%), drowsiness (6%), dizziness (7%), sweating.
Incidence 1% or Less
Body as a Whole: weight gain, fever, infections, asthenia.
Cardiovascular: palpitation, pallor, syncope.
Dermatologic: urticaria.
Gastrointestinal: gastritis, rectal bleeding, eructation, anorexia, increased appetite.
Hemic and Lymphatic: epistaxis, anemia, eosinophilia.
Nervous System: tremors, abnormal dreams, hallucinations, euphoria, extrapyramidal symptoms, vertigo, paresthesia, depression, insomnia, nervousness, excessive thirst, dry mouth, abnormal thinking, inability to concentrate, hyperkinesis, stupor.
Respiratory: dyspnea, pulmonary edema, rhinitis, cough.
Special Senses: abnormal taste, abnormal vision, blurred vision, tinnitus, hearing loss.
Urogenital: hematuria, proteinuria, oliguria, urinary retention, polyuria, increased urinary frequency.
The following adverse events were reported from postmarketing experience.
Body as a Whole: hypersensitivity reactions such as anaphylaxis, anaphylactoid reaction, laryngeal edema, tongue edema (see Boxed WARNING, WARNINGS), myalgia.
Cardiovascular: hypotension and flushing.
Dermatologic: Lyell's syndrome, Stevens-Johnson syndrome, exfoliative dermatitis, maculo-papular rash, urticaria.
Gastrointestinal: peptic ulceration, GI hemorrhage, GI perforation (see Boxed WARNING, WARNINGS), melena, acute pancreatitis.
Hemic and Lymphatic: postoperative wound hemorrhage, rarely requiring blood transfusion (see Boxed WARNING, WARNINGS and PRECAUTIONS), thrombocytopenia, leukopenia.
Hepatic: hepatitis, liver failure, cholestatic jaundice.
Nervous System: convulsions, psychosis, aseptic meningitis.
Respiratory: asthma, bronchospasm.
Urogenital: acute renal failure (see Boxed WARNING, WARNINGS), flank pain with or without hematuria and/or azotemia, nephritis, hyponatremia, hyperkalemia, hemolytic uremic syndrome.
OVERDOSAGE
In controlled overdosage, daily doses of 360 mg of ketorolac tromethamine-IV/IM given for five days (3 times the highest recommended dose), caused abdominal pain and peptic ulcers which healed after discontinuation of dosing. Metabolic acidosis has been reported following intentional overdosage.
Dialysis does not significantly clear ketorolac tromethamine from the blood stream.
DOSAGE AND ADMINISTRATION
THE COMBINED DURATION OF USE OF KETOROLAC TROMETHAMINE-IV/IM AND KETOROLAC TROMETHAMINE TABLETS IS NOT TO EXCEED FIVE (5) DAYS. THE USE OF KETOROLAC TROMETHAMINE TABLETS IS ONLY INDICATED AS CONTINUATION THERAPY TO KETOROLAC TROMETHAMINE-IV/IM.
Ketorolac tromethamine-IV/IM may be used as a single, or multiple dose, on a regular or "prn" schedule for the management of moderately severe, acute pain that requires analgesia at the opioid level, usually in a post operative setting. Hypovolemia should be corrected prior to the administration of ketorolac tromethamine (see WARNINGS: Renal Effects). Patients should be switched to alternative analgesics as soon as possible, but ketorolac tromethamine therapy is not to exceed 5 days.
Ketorolac Tromethamine Tablets are indicated ONLY as continuation therapy to ketorolac tromethamine-IV/IM for the management of moderately severe, acute pain that requires analgesia at the opioid level. See also PRECAUTIONS: Information for Patients.
Transition from Ketorolac Tromethamine-IV/IM to Ketorolac Tromethamine Tablets
The recommended dose for ketorolac tromethamine tablets is as follows:
Patients < 65 years of age
Two (2) tablets as a first oral dose for patients who received 60 mg IM single dose, 30 mg IV single dose or 30 mg multiple dose ketorolac tromethamine-IV/IM followed by one (1) tablet of ketorolac tromethamine orally every 4 to 6 hours, not to exceed 40 mg/24h of oral ketorolac tromethamine.
Patients ≥ 65 years of age, renally impaired and/or less than 50 kg (110 lbs) of body weight
One (1) tablet as a first oral dose for patients who received 30 mg IM single dose, 15 mg IV single dose or 15 mg multiple dose ketorolac tromethamine-IV/IM followed by one (1) tablet of ketorolac tromethamine orally every 4 to 6 hours, not to exceed 40 mg/24h of oral ketorolac tromethamine.
Shortening the recommended dosing intervals may result in increased frequency and severity of adverse reactions.
The maximum combined duration of use (parenteral and oral ketorolac tromethamine) is limited to 5 days.
HOW SUPPLIED
Ketorolac Tromethamine Tablets, USP are available containing 10 mg of ketorolac tromethamine. The tablets are film-coated, white, unscored round tablets debossed with M over 134 on one side and blank on the other side. They are available as follows:
NDC 0378-1134-01
bottles of 100 tablets
STORE AT CONTROLLED ROOM TEMPERATURE 20°–25°C (68°–77°F). [See USP]
Dispense in a tight container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REVISED JULY 2003
KTLC:R5
| Ketorolac Tromethamine (Ketorolac Tromethamine) | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Revised: 08/2007Mylan Pharmaceuticals Inc.