SUMYCIN
-
tetracycline syrup
Par Pharmaceutical, Inc.
----------
SUMYCIN® SYRUPTETRACYCLINE ORAL SUSPENSION, USP
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Sumycin Syrup (Tetracycline Oral Suspension, USP) and other antibacterial drugs, Sumycin Syrup (Tetracycline Oral Suspension, USP) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
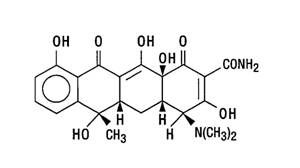
Sumycin for oral administration contains tetracycline, an antibiotic isolated from Streptomyces aureofaciens. Tetracycline is described chemically as 4-(dimethylamino)-1, 4, 4a, 5, 5a, 6, 11, 12a-octahydro-3, 6, 10, 12, 12a-pentahydroxy-6-methyl-1, 11-dioxo-2-napthacenecarboxamide; its structural formula is:
Sumycin Syrup (Tetracycline Oral Suspension, USP) is a suspension containing, in each 5 mL teaspoonful, tetracycline equivalent to 125 mg tetracycline hydrochloride. Inactive ingredients: citric acid, colorant (D&C Yellow No. 10), flavor, potassium citrate, potassium metaphosphate, purified water, saccharin sodium, sodium benzoate, sodium citrate, sodium metabisulfite, sorbitol solution, sucrose, and tragacanth.
CLINICAL PHARMACOLOGY
Tetracyclines are adequately but incompletely absorbed from the gastrointestinal tract. Approximately 65 percent of a short-acting tetracycline is bound to plasma proteins; the plasma protein binding for intermediate- and long-acting analogues is usually greater.
Penetration of the tetracyclines into most body fluids and tissues is excellent. Tetracyclines are distributed in varying degrees into bile, liver, lung, kidney, prostate, urine, cerebrospinal fluid, synovial fluid, mucosa of the maxillary sinus, brain, sputum, and bone. Tetracyclines cross the placenta and enter the fetal circulation and amniotic fluid.
Following a single oral dose, peak plasma concentrations are achieved in two to four hours.
Tetracyclines are concentrated by the liver in the bile. They are excreted in both the urine and feces at high concentrations in a biologically active form. Since renal clearance of tetracyclines is by glomerular filtration, excretion is significantly affected by the state of renal function. (See WARNINGS.)
The tetracyclines are primarily bacteriostatic and are thought to exert their antimicrobial effect by the inhibition of protein synthesis. The tetracyclines have a similar antimicrobial spectrum of activity against a wide range of gram-positive and gram-negative organisms. Cross-resistance of these organisms to tetracyclines is common. In addition, gram-negative bacilli made tetracycline-resistant, may also show cross-resistance to chloramphenicol.
Calymmatobacterium granulomatis
Because many strains of the following groups of gram-negative microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are especially recommended:
Enterococcus group [Enterococcus faecalis (formerly Streptococcus faecalis) and Enterococcus
faecium (formerly Streptococcus faecium)]
Because many strains of these gram-positive microorganisms have been shown to be resistant to tetracycline, culture and susceptibility testing are recommended. Up to 44 percent of strains of Streptococcus pyogenes and 74 percent of Enterococcus faecalis (formerly Streptococcus faecalis) have been found to be resistant to tetracycline drugs. Therefore, tetracyclines should not be used for treatment of streptococcal disease unless the organism is known to be susceptible.
Quantitative methods that require measurement of zone diameters give the most precise estimate of the susceptibility of bacteria to antimicrobial agents. One such standard procedure1 that has been recommended for use with disks to test susceptibility of microorganisms to tetracycline uses the 30-mcg tetracycline disk. Interpretation involves the correlation of the zone diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for tetracycline.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 30-mcg tetracycline disk should be interpreted according to the following criteria:
| Zone diameter (mm) | Interpretation |
| ≥ 19 | Susceptible |
| 15 – 18 | Intermediate |
| ≤ 14 | Resistant |
A report of “Susceptible” indicates that the pathogen is likely to be inhibited by generally achievableblood levels. A report of “Intermediate” suggests that the organism would be susceptible if high dosage isused or if the infection is confined to tissues or fluids in which high antibiotic (or antimicrobial) levels are attained. A report of “Resistant” indicates that achievable concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 30-mcg tetracycline disk should give the following zone diameters:
| Organism | - | Zone Diameter (mm) |
| E. coli | ATCC25922 | 18 – 25 |
| S. aureus | ATCC25923 | 19 - 18 |
Use a standardized dilution method2 (broth, agar, microdilution) or equivalent with tetracycline powder. The MIC values obtained should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation |
| ≤ 4.0 | Susceptible |
| > 4.0 < 16 | Intermediate |
| ≥ 16 | Resistant |
As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard tetracycline powder should provide the following MIC values:
| Organism | - | Zone Diameter (mm) |
| E. coli | ATCC25922 | 1 – 4 |
| S. aureus | ATCC29213 | 0.25 – 1 |
| E. faecalis | ATCC29212 | 8 – 32 |
| P. aeruginosa | ATCC27853 | 8 - 32 |
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Sumycin Syrup (Tetracycline Oral Suspension, USP) and other antibacterial drugs, Sumycin Syrup (Tetracycline Oral Suspension, USP) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Tetracycline hydrochloride is indicated for the treatment of the following infections:
Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsial pox and tick fevers caused by Rickettsiae
Respiratory tract infections caused by Mycoplasma pneumoniae
Lymphogranuloma venereum caused by Chlamydia trachomatis
Psittacosis and ornithosis due to Chlamydia psittaci
Trachoma caused by Chlamydia trachomatis, although the infectious agent is not always eliminated, as judged by immunofluorescence
Inclusion conjunctivitis caused by Chlamydia trachomatis
Tetracycline hydrochloride is indicated for the treatment of uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydia trachomatis
Nongonococcal urethritis caused by Ureaplasma urealyticum
Relapsing fever due to Borrelia recurrentis
Tetracycline hydrochloride is also indicated for the treatment of infections caused by the following gramnegative microorganisms:
Chancroid caused by Haemophilus ducreyi
Plague due to Yersinia pestis (formerly Pasteurella pestis)
Tularemia due to Francisella tularensis (formerly Pasteurella tularensis)
Cholera caused by Vibrio cholerae (formerly Vibrio comma)
Campylobacter fetus infections caused by Campylobacter fetus (formerly Vibrio fetus)
Brucellosis due to Brucella species (in conjunction with streptomycin)
Bartonellosis due to Bartonella bacilliformis
Granuloma inguinale caused by Calymmatobacterium granulomatis
Because many strains of the following groups of microorganisms have been shown to be resistant to tetracycline hydrochloride, culture and susceptibility testing are recommended.
Tetracycline hydrochloride is indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug:
Enterobacter aerogenes (formerly Aerobacter aerogenes)
Acinetobacter species (formally Mima species and Herellea species)
Respiratory tract infections caused by Haemophilus influenzae
Respiratory tract and urinary tract infections caused by Klebsiella species
Tetracycline hydrochloride is indicated for treatment of infections caused by the following gram-positive microorganisms when bacteriologic testing indicated appropriate susceptibility to the drug:
For upper respiratory infections caused by Streptococcus pneumoniae (formerly Diplococcus pneumoniae).
Skin and skin structure infections caused by Staphylococcus aureus.
Tetracyclines are not the drugs of choice in the treatment of any type of staphylococcal infections.
When penicillin is contraindicated, tetracycline hydrochloride is an alternative drug in the treatment of the following infections:
Uncomplicated gonorrhea caused by Neisseria gonorrhoeae
Syphilis caused by Treponema pallidum
Yaws caused by Treponema pertenue
Listeriosis due to Listeria monocytogenes
Anthrax due to Bacillus anthracis
Vincent’s infection caused by Fusobacterium fusiforme
Actinomycosis caused by Actinomyces israelii
Infections caused by Clostridia species
In acute intestinal amebiasis, the tetracycline hydrochlorides may be a useful adjunctive therapy to amebicides.
In severe acne the tetracycline hydrochlorides may be useful adjunctive therapy.
CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
WARNINGS
TETRACYCLINE-CLASS ANTIBIOTICS CAN CAUSE FETAL HARM WHEN ADMINISTERED TO A PREGNANT WOMAN. IF ANY TETRACYCLINE IS USED DURING PREGNANCY, OR IF THE PATIENT BECOMES PREGNANT WHILE TAKING THESE DRUGS, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS.
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY AND CHILDHOOD TO AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN).
This adverse reaction is more common during long term use of the drug but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED DURING TOOTH DEVELOPMENT UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED.
All tetracyclines form a stable calcium complex in any bone forming tissues. A decrease in fibula growth rate has been observed in young animals (rats and rabbits) given oral tetracycline in doses of 25 mg/kg every six hours. This reaction was shown to be reversible when the drug was discontinued.
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy.
Sumycin Syrup (Tetracycline Oral Suspension, USP) contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
The antianabolic action of tetracycline may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, even usual oral or parenteral dose may lead to excessive systemic accumulation of the drug and possible liver toxicity. Under such conditions, lower than usual doses are indicated and, if therapy is prolonged, serum level determinations of the drug may be advisable.
Photosensitivity, manifested by an exaggerated sunburn reaction, has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultra-violet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema. NOTE: Photosensitization reactions have occurred most frequently with demeclocycline, less with chlortetracycline, and very rarely with oxytetracycline and tetracycline.
GENERAL PRECAUTIONS
Prescribing Sumycin Syrup (Tetracycline Oral Suspension, USP) in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
As with other antibiotics, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate therapy instituted. NOTE: Superinfection of the bowel by staphylococci may be life-threatening. Pseudotumor cerebri (benign intracranial hypertension) in adults has been associated with the use of tetracyclines. The usual clinical manifestations are headache and blurred vision. Bulging fontanels have been associated with the use of tetracyclines in infants. While both of these conditions and related symptoms usually resolve after discontinuation of the tetracycline, the possibility for permanent sequelae exists.
Since sensitivity reactions are more likely to occur in persons with a history of allergy, asthma, hay fever, or urticaria, the preparation should be used with caution in such individuals.
Cross-sensitization among the various tetracyclines is extremely common.
Incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy, when indicated.
Under no circumstances should outdated tetracyclines be administered, as the degradation of tetracyclines are highly nephrotoxic and have, on occasion, produced a Fanconi-like syndrome.
Information for Patients
Patients should be counseled that antibacterial drugs including Sumycin Syrup (Tetracycline Oral Suspension, USP) should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Sumycin Syrup (Tetracycline Oral Suspension, USP) is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Sumycin Syrup (Tetracycline Oral Suspension, USP) or other antibacterial drugs in the future.
Laboratory Tests
During long-term therapy, periodic laboratory evaluation of organ system function, including renal, hepatic, and hematopoietic systems, should be performed.
All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with tetracycline should have a follow-up serologic test for syphilis after 3 months.
Drug Interactions
PENICILLIN - Since bacteriostatic drugs like tetracycline may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline in conjunction with penicillin.
ANTICOAGULANTS - Because the tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
ANTACIDS AND IRON CONTAINING PRODUCTS - Absorption of tetracycline is impaired by antacids containing aluminum, calcium, or magnesium, and iron containing preparations.
ORAL CONTRACEPTIVES - Concurrent use of tetracycline may render oral contraceptives less effective.
METHOXYFLURANE - The concurrent use of tetracycline and methoxyflurane has been reported to result in fatal renal toxicity.
Carcinogenesis and Mutagenesis and Impairment of Fertility
Long-term studies conducted in rats and mice to determine whether tetracycline hydrochloride has carcinogenic potential were negative. Some related antibiotics (oxytetracycline, minocycline) have shown evidence of oncogenic activity in rats.
In two in vitro mammalian cell assay systems (L51784y mouse lymphoma and Chinese hamsterlung cells), there was evidence of mutagenicity at tetracycline hydrochloride concentrations of 60 and 10 μg/mL, respectively.
Tetracycline hydrochloride had no effect on fertility when administered in the diet to male and female rats at a daily intake of 25 times the human dose.
Pregnancy: Teratogenic effects: Pregnancy Category D (see WARNINGS.)
Pregnancy: Nonteratogenic effects: (see WARNINGS.)
Labor and Delivery
The effect of tetracyclines on labor and delivery is unknown.
Nursing Mothers
Tetracyclines are present in the milk of lactating women who are taking a drug in this class. Because of the potential for serious adverse reactions in nursing infants from tetracyclines, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see WARNINGS.)
Pediatric Use
See WARNINGS and DOSAGE AND ADMINISTRATION.
ADVERSE REACTIONS
Anorexia, epigastric distress, nausea, vomiting, diarrhea, bulky loose stools, stomatitis, sore throat, glossitis, black hairy tongue, dysphagia, hoarseness, enterocolitis, and inflammatory lesions (with candidal overgrowth) in the anogenital region, including proctitis and pruritus ani. Rare instances of esophagitis and esophageal ulceration have been reported in patients receiving particularly the capsule and also the tablet forms of tetracyclines. Most of the patients were reported to have medication immediately before going to bed (see DOSAGE AND ADMINISTRATION). These reactions have been caused by both the oral and parenteral administration of tetracyclines but are less frequent after parenteral use.
Skin and Skin Structures: maculopapular and erythematous rashes.
Exfoliative dermatitis has been reported but is uncommon. Onycholysis and discoloration of the nails have been reported rarely. Photosensitivity has occurred. (See WARNINGS.)
Increases in BUN have been reported and are apparently dose-related. (See WARNINGS.)
Hepatic cholestatis has been reported rarely, and is usually associated with high dosage levels of tetracycline.
Anaphylaxis; serum sickness-like reactions, as fever, rash, and arthralgia; urticaria, angioneurotic edema, anaphylactoid purpura, pericarditis, exacerbation of systemic lupus erythematosus.
Blood: anemia, hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, neutropenia and eosinophilia have been reported.
Dizziness and headache have been reported.
When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function are known to occur. Bulging fontanels in infants and intracranial hypertension in adults have been reported. (See PRECAUTIONS—General.)
OVERDOSAGE
In case of overdosage, treat symptomatically and institute supportive measures.
DOSAGE AND ADMINISTRATION
Adults: usual daily dose is 1 to 2 g: for mild to moderate infections: 500 mg bid or 250 mg qid; higher dosages such as 500 mg qid may be required for severe infections.
For children above eight years of age: usual daily dose is 10 to 20 mg/lb (25 to 50 mg/kg) body weight divided in four equal doses.
Representative pediatric dosages for the syrup on a qid basis are as follows:
| 20 lbs | 2.5 mL | (1/2 teaspoonful) |
| 40 lbs | 5 mL | (1 teaspoon) |
| 60 lbs | 7.5 mL | (1–1/2 teaspoonfuls) |
| 80 lbs | 10 Ml | (2 teaspoonfuls) |
Therapy should be continued for at least 24 to 48 hours after symptoms and fever have subsided.
The treatment of brucellosis, 500 mg tetracycline four times daily for three weeks should be accompanied by streptomycin, 1 g intramuscularly twice daily the first week and once daily the second week.
For treatment of uncomplicated gonorrhea, 500 mg every six hours for seven days.
For treatment of syphilis, a total of 30 to 40 g in equally divided doses over a period of 10 to 15 days should be given. Close follow up, including laboratory tests, is recommended.
Uncomplicated urethral, endocervical, or rectal infection in adults caused by Chlamydia trachomatis: 500 mg by mouth, four times a day for at least seven days.
In cases of severe acne which in the judgment of the clinician, requires long-term treatment, the recommended initial dosage is 1 g daily in divided doses. When improvement is noted, usually within one week, dosage should be gradually reduced to maintenance levels ranging from 125 to 500 mg daily. In some patients it may be possible to maintain adequate remission of lesions with alternate-day or intermittent therapy. Tetracycline therapy of acne should augment the other standard measures known to be of value.
In patients with renal impairment (see WARNINGS) total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses.
In the treatment of streptococcal infections, a therapeutic dose of tetracycline should be administered for at least 10 days.
Concomitant therapy: Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium, and iron containing preparations.
Food and some dairy products also interfere with absorption.
HOW SUPPLIED
Sumycin Syrup (Tetracycline Oral Suspension, USP) is available as a fruit-flavored suspension containing, in each 5 mL teaspoonful, tetracycline equivalent to 125 mg tetracycline hydrochloride. NDC 49884-799-33 Bottles of 473 mL (16 fl. oz.)
Keep tightly closed. Protect from light. Store below 30º C (86º F).
ANIMAL PHARMACOLOGY AND/OR TOXICOLOGY
Hyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO4 and methacycline; in minipigs by doxycycline, minocycline, tetracycline PO4 and methacycline; in dogs by doxycycline and minocycline; in monkeys by minocycline.
Minocycline, tetracycline PO4, methacycline, doxycycline, tetracycline base, oxytetracycline HCl and tetracycline HCl were goitrogenic in rats fed a low iodine diet. This goitrogenic effect was accompanied by high radioactive iodine uptake. Administration of minocycline also produced a large goiter with high radioiodine uptake in rats fed a relatively high iodine diet.
Treatment of various animal species with this class of drugs has also resulted in the induction of thyroid hyperplasia in the following: in rats and dogs (minocycline), in chickens (chlortetracycline), and in rats and mice (oxytetracycline). Adrenal gland hyperplasia has been observed in goats and rats treated with oxytetracycline.
REFERENCES
1. National Committee for Clinical Laboratory Standards, Performance Standards for Antimicrobial Disk Susceptibility Tests–Fourth Edition. Approved Standard NCCLS Document M2-A4, Vol. 10, No. 7 NCCLS, Villanova, PA, April 1990.
2. National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically–Second Edition. Approved Standard NCCLS Document M7-A2, Vol. 10, No. 8 NCCLS, Villanova, PA, April 1990.
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
| SUMYCIN
tetracycline syrup |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA060400 | 03/31/2004 | 04/30/2008 |
| Labeler - Par Pharmaceutical, Inc. (092733690) |
| Registrant - Par Pharmaceutical Inc. (092733690) |