ARDUAN
-
pipecuronium bromide injection
Organon Pharmaceuticals USA
----------
ARDUAN®(pipecuronium bromide) for injection
THIS DRUG SHOULD BE ADMINISTERED BY ADEQUATELY-TRAINED INDIVIDUALS FAMILIAR WITH ITS ACTIONS, CHARACTERISTICS, AND HAZARDS.
DESCRIPTION
ARDUAN® (pipecuronium bromide) for injection is a long-acting non-depolarizing neuromuscular blocking agent, chemically designated as piperazinium, 4, 4´-[(2β, 3α, 5α, 16β, 17β)-3, 17-bis (acetyloxy) androstane-2, 16-diyl] bis [1, 1-dimethyl]-, dibromide, dihydrate.
The structural formula is:
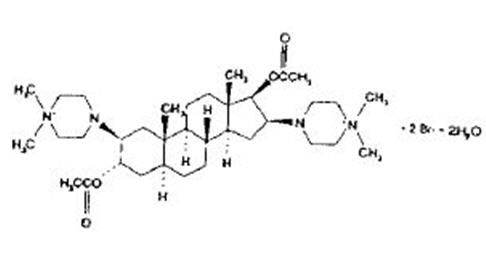
The chemical formula is C35H62N4O4Br2 • 2H2O with a molecular weight of 798.74. At normal physiological states, the compound exists primarily in the ionized form and is poorly soluble in fat.
ARDUAN® is supplied as a sterile nonpyrogenic freeze-dried cake, for intravenous injection only. Each 10 mL vial contains 10 mg pipecuronium bromide, and 380 mg mannitol, USP (to adjust tonicity). When necessary, pH is adjusted with sodium hydroxide and/or hydrochloric acid (pH 6).
Bacteriostatic water for injection, USP, when supplied, contains 0.9% w/v BENZYL ALCOHOL, WHICH IS NOT FOR USE IN NEWBORNS.
CLINICAL PHARMACOLOGY
ARDUAN® (pipecuronium bromide) for injection is a long-acting non-depolarizing neuromuscular blocking agent possessing all of the characteristic pharmacological actions of this class of drugs (curariform). It acts by competing for cholinergic receptors at the motor end-plate. This action is antagonized by acetylcholinesterase inhibitors, such as neostigmine.
Pharmacodynamics
The individual cumulative ED95 (dose required to produce 95% suppression of T1 of the train-of-four or 95% suppression of single twitch response) during balanced anesthesia has averaged 41 µg/kg actual body weight (ABW) (range 20–91 µg/kg). Maximum blockade is achieved in approximately 5 minutes following single doses of 70 to 85 µg/kg ABW. Under balanced anesthesia, following single doses of 70 µg/kg ABW in 4 clinical trials (n = 65), the mean times to recovery to 25% of control (clinical duration) were 47–98 minutes (range 30–175 min.). The mean times to recovery following 80–85 µg/kg ABW single doses in 4 clinical trials (n = 69) were 80–124 minutes (range 40–211 min.). ARDUAN® was shown to have an onset time and clinical duration (range and variability) similar to those of pancuronium bromide at comparable doses (historic data and limited comparisons).
An analysis of 282 cases in U.S. clinical trials utilizing a variety of premedications, varying lengths of surgery, and various anesthetic agents, indicates that two-thirds of the patients had clinical durations within 30 minutes of the duration predicted by the dose adjusted by ideal body weight (IBW) and calculated creatinine clearance (there is an inverse relationship between renal function and clinical duration such that the mean clinical duration more than doubles when the calculated creatinine clearance goes from 100 to 40 mL/min.).
The likelihood of prolonged clinical duration may be decreased by calculating creatinine clearance based on serum creatinine and ideal body weight based on height, or by using doses at the lower end of the recommended dosage range for intubation in patients with moderate decreases in renal function. In patients with renal failure the drug should be used with caution (see Table II, Individualization of Dosage Subsection of CLINICAL PHARMACOLOGY, and PRECAUTIONS).
In 333 of nearly 600 cases in U.S clinical trials with evaluable clinical duration data, clinical durations greater than 120 minutes for the dose of 70 µg/kg ABW or greater than 150 minutes for doses of 80 µg/kg ABW or more were reported in approximately 8% (27/333) of cases. In about one-third (10/27) of such cases, dosage was administered to obese patients (defined as 30% or more above ideal body weight for height) based on actual body weight. Prolonged clinical duration was approximately 2 times more common in obese patients (10/73 cases) than among non-obese (17/260 cases) patients. Therefore, in calculating dosage on a mg/kg basis, ideal body weight can also be used to decrease the variability in clinical duration and to reduce the possibility of overdosage in the obese population (see Individualization of Dosage Subsection of CLINICAL PHARMACOLOGY).
Limited data (3 studies, n =29) are available on the administration of single doses of 100 µg/kg ABW of ARDUAN®. No significant differences were observed in mean clinical duration compared to that seen with doses of 80–85 µg/kg ABW. Doses above 100 µg/kg based on actual or ideal body weight are not recommended because of the possibility of even longer duration of action for individual patients.
The mean time for spontaneous recovery from 25% to 50% of control T1, based on 90 patients in 6 studies in whom the final dose produced a T1 less than 25% of control, is approximately 24 minutes (range 8–131 minutes). Because of the use of antagonism following surgery, there are insufficient data to report the time required for greater than 50% spontaneous recovery of T1.
ARDUAN® can be administered following recovery from succinylcholine, when the latter is used to facilitate endotracheal intubation. Preliminary data (from 1 study in 25 patients) suggest that, if a single dose of 50 µg/kg ABW ARDUAN® is administered under these conditions, prolongation in clinical duration may be noted (range of 23–95 minutes following succinylcholine versus 8–50 minutes without it). Prior use of succinylcholine has not been shown to alter the clinical duration of larger doses of ARDUAN® (80 or more µg/kg ABW administered after recovery from succinylcholine, n =53).
Initial ARDUAN® doses of 70 to 85 µg/kg ABW, used without succinylcholine (from 3 studies, in 43 patients), have produced good to excellent intubation conditions within 2.5 to 3.0 minutes of injection (which is before maximum blockade). Review of the time (range from 2–6 minutes) to intubation and any comments about the quality of intubation in patients receiving up to 100 µg/kg ABW indicated no reports of problems in intubating such patients.
The mean clinical duration of first maintenance doses of 10–15 µg/kg ABW ARDUAN® administered at 25% recovery of control T1 is approximately 50 minutes (range 17–175 minutes: 6 studies, 148 patients).
Hemodynamics
Administration of ARDUAN® doses up to and including 100 µg/kg (= 2.5 × ED95) as a rapid bolus over 5 sec. to healthy patients during stable state balanced anesthesia produced no dose-related effects on heart-rate or blood pressure.
In patients undergoing surgery for coronary artery bypass grafting, hemodynamic studies were performed using higher than currently recommended ARDUAN® doses, i.e., 100 µg/kg ABW and 200 µg/kg ABW. ARDUAN® was administered during induction of anesthesia with a narcotic or etomidate/narcotic combination, respectively. Observed hemodynamic effects were small and included reductions in mean systolic and mean arterial pressures (↓ 10–14%), ventricular stroke-work index (↓ 8–25%) and cardiac output (↓ 20%); sustained increases in pulmonary capillary wedge and central venous pressures and changes in mean heart rate were not observed. ARDUAN® has not been studied in patients with hemodynamic dysfunction secondary to cardiac valvular disease.
ARDUAN® has not been found to influence the cardiovascular depression or stimulations associated with other drugs administered during anesthesia or with surgical stimulation. The most common observations, comparing vital signs immediately prior to initial dosage with ARDUAN® and two minutes after injection, are a slight decrease in heart rate, systolic blood pressure and diastolic blood pressure.
Human plasma histamine concentrations, following effective initial doses of ARDUAN®, have not been studied. However, clinical experience with more than 1000 patients indicates hypersensitivity reactions such as bronchospasm, tachycardia, and other reactions commonly associated with histamine release are unlikely to occur.
Pharmacokinetics
Only limited information is available, at the present time, regarding the pharmacokinetics of ARDUAN® in humans. Preliminary pharmacokinetic results from 4 normal subjects and 7 subjects undergoing cadaver renal transplant is reproduced in Table I. These results tend to indicate that some prolongation of plasma levels can be expected in patients with severe impairment of renal function which may in turn significantly extend recovery time. A clear relationship between plasma levels and the degree or extent of muscle twitch suppression has not been determined at this time.
| Normal Renal and Hepatic Function N=4 | Renal Transplant N=7 |
|
|---|---|---|
|
||
| Clearance (L/hr/kg) | 0.12 (0.10–0.14) | 0.08 (0.02–0.12) |
| Volume of Distribution at Steady State (L/kg) | 0.25 (0.12–0.37) | 0.37 (0.28–0.51) |
| t1/2 distribution (min) | 6.22 (1.34–10.66) | 4.33 (1.69–6.17) |
| t1/2 elimination (hr) | 1.7 (0.9–2.7) | 4.0 (2.0–8.2) |
Studies of distribution, metabolism, and excretion in animals (rats, dogs, and cats), indicate that ARDUAN® is eliminated primarily by the kidneys (more than 75% of drug recovered in the urine, primarily as the unchanged drug). The 3-deacetyl, 17-deacetyl, and 3,17-dideacetyl derivatives of ARDUAN® have been identified in urine collected from dogs; these metabolites account for approximately 20% of the administered dose. The 3-deacetyl derivative is the only metabolite with substantial neuromuscular blocking activity, manifesting approximately 40–50% of the activity of the parent drug in the cat and the dog. On the basis of experience gained with other agents in this category it is most probable that similar metabolites exist in other animal species and in humans.
At the present time only the 3-deacetyl metabolite of ARDUAN® has been detected in the urine of humans undergoing coronary artery bypass surgery. Following administration of 200 µg/kg ABW, 56% of the administered dose was recovered in the urine, of which 41% was unchanged drug and the remaining 15% was the 3-deacetyl metabolite of ARDUAN®. In the same study no metabolites of ARDUAN® were found in the plasma.
Individualization of Dosage
ARDUAN® like other long-acting neuromuscular blocking agents, displays a great deal of variability in the clinical duration of its effect. With experience, anesthesiologists will determine when and how to modify dosage on individual patients based on clinical factors like age, sex, weight/degree of obesity, renal, hepatic and/or other diseases, etc., much as they do with pancuronium. Tables II and III are included in the insert to assist those physicians who may wish to adjust dosage based on ideal body weight and renal function, two factors which were identified in controlled clinical trials that may warrant dosage adjustment. Although the tables differ in approach and thus appear different, i.e., one derives the total dose in mg and the other presents it as µg/kg IBW, they ultimately suggest the same dose for patients with the same height, weight, age and serum creatinine level.
It should be noted from the tables that for small patients with decreased renal function the suggested initial dose is less than 70 to 85 µg/kg IBW, i.e., less than 2x the average ED95 dose which is generally the recommended intubating dose for neuromuscular blocking agents. A review of 80 patients who received initial doses <70µg/kg IBW compared with 202 who received >70 µg/kg doses in controlled clinical trials showed neither longer mean time to intubation nor any problems during intubation. HOWEVER, PHYSICIANS SHOULD USE EXTRA CARE DURING INTUBATION OF ANY PATIENT IN WHOM, IN ORDER TO DECREASE THE POSSIBILITY OF PROLONGED CLINICAL DURATION, THEY ELECT TO USE LESS THAN 70 µG/KG IBW FOR INTUBATION.
Dosing in accordance with the following tables may reduce the variability in clinical duration to bring approximately 20% more patients to within ± 30 minutes of the duration predicted by the dose adjusted by IBW and calculated creatinine clearance. It should be emphasized, however, that it will not entirely eliminate the variability associated with the use of long acting neuromuscular blocking agents and physicians must be prepared to monitor patients carefully during surgery and recovery to support them until they have adequate return of muscular function.
| Based on Ideal Body Weight in kg* and Estimated Creatinine Clearance†
(mg = mL if 10 mg vial is reconstituted with 10 mL) |
||||||||
| Creatinine Clearance | ||||||||
| mL/min | kg IBW | 50 | 60 | 70 | 80 | 90 | 100 | kg IBW |
| < = 40 | (2.5) | (3.0) | (3.5) | (4.0) | (4.5) | (5.0) | ||
| 60 | (2.5) | (3.0) | 3.8 | 4.9 | 6.2 | 7.7 | ||
| 80 | 2.6 | 3.7 | 5.0 | 6.5 | 8.3 | (10.0) | ||
| > = 100 | 3.2 | 4.6 | 6.3 | 8.2 | (9.0) | (10.0) | ||
| mL/min | ||||||||
| ( ) Minimum calculated dose for adequate intubation – in these patients prolonged clinical blockade should be anticipated. [ ] Maximum calculated dose for intubation – in these patients use of maintenance doses should be anticipated. NOTE: Use actual body weight in the calculation if it is less than ideal body weight. |
||||
| Ideal Body Weight*
Adjusted for Renal Function (Estimated Creatine Clearance †) |
||||
| Creatinine Clearance in mL/min ≥ | ||||
| <40 | 60 | 80 | 100 | >100 |
| (50) | 55 | 70 | 85 | [100] |
| --- µg/kg ideal body wt. --- | ||||
INDICATIONS AND USAGE
ARDUAN® (pipecuronium bromide) for injection is a long-acting neuromuscular blocking agent, indicated as an adjunct to general anesthesia, to provide skeletal muscle relaxation during surgery. ARDUAN® can also be used to provide skeletal muscle relaxation for endotracheal intubation.
CONTRAINDICATIONS
None known.
WARNINGS
ARDUAN® (PIPECURONIUM BROMIDE) FOR INJECTION SHOULD BE ADMINISTERED IN CAREFULLY ADJUSTED DOSAGE BY OR UNDER THE SUPERVISION OF EXPERIENCED CLINICIANS WHO ARE FAMILIAR WITH THE DRUG'S ACTIONS AND THE POSSIBLE COMPLICATIONS OF ITS USE. THE DRUG SHOULD NOT BE ADMINISTERED UNLESS FACILITIES FOR INTUBATION, ARTIFICIAL RESPIRATION, OXYGEN THERAPY, AND AN ANTAGONIST ARE WITHIN IMMEDIATE REACH. IT IS RECOMMENDED THAT CLINICIANS ADMINISTERING LONG-ACTING NEUROMUSCULAR BLOCKING AGENTS SUCH AS ARDUAN® EMPLOY A PERIPHERAL NERVE STIMULATOR TO MONITOR DRUG RESPONSE, NEED FOR ADDITIONAL RELAXANT, AND ADEQUACY OF SPONTANEOUS RECOVERY OR ANTAGONISM.
Anaphylaxis
Severe anaphylactic reactions to neuromuscular blocking agents, including ARDUAN®, have been reported. These reactions have, in some cases, been life-threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those individuals who have had previous anaphylactic reactions to other neuromuscular blocking agents, since cross-reactivity between neuromuscular blocking agents, both depolarizing and non-depolarizing, has been reported.
In patients with myasthenia gravis or myasthenic (Eaton-Lambert) syndrome, small doses of non-depolarizing neuromuscular blocking agents may have profound effects. Shorter acting muscle relaxants than ARDUAN® may be more suitable for these patients.
PRECAUTIONS
General
Since ARDUAN® (pipecuronium bromide) for injection has little or no effect on the heart rate, the drug will not counteract the bradycardia produced by many opioid anesthetic agents or vagal stimulation.
Anaphylaxis
Since allergic cross-reactivity has been reported in this class, request information from your patients about previous anaphylactic reactions to other neuromuscular blocking agents. In addition, inform your patients that severe anaphylactic reactions to neuromuscular blocking agents, including ARDUAN®, have been reported.
Renal Failure
ARDUAN® in the dose of 70 µg/kg ABW, has been studied in a limited number of patients (n=20) undergoing renal transplant surgery, recently dialyzed in preparation for cadaver renal transplant. The mean clinical duration (injection to 25% recovery) of 103 minutes was not judged prolonged, however, there was wide individual variation (30 to 267 minutes). ARDUAN® has not otherwise been studied in patients with renal failure (for elective or emergency non-renal surgery). Because it is primarily excreted by the kidney, ARDUAN® should be used with caution in patients with renal failure (see Table II, Individualization of Dosage Subsection of CLINICAL PHARMACOLOGY).
Increased Volume of Distribution
Conditions associated with an increased volume of distribution, e.g., slower circulation time in cardiovascular disease, old age or edematous states, may be associated with a delay in onset time. Because higher doses of ARDUAN® may produce a longer duration of action, the initial dosage should not usually be increased in these patients to enhance onset time; instead, more time should be allowed for the drug to achieve maximum effect.
Hepatic Disease
There are no data on dosage requirements, onset, duration or pharmacokinetics in patients with moderate or severe hepatic dysfunction and/or biliary obstruction. This should be considered in selection of muscle relaxants for use in these patients.
Obesity
The most common patient condition associated with prolonged clinical duration was obesity, defined as 30% or more over ideal body weight (see CLINICAL PHARMACOLOGY). Clinical study subjects were dosed on the basis of actual body weight, which may have contributed to the higher incidence of prolonged duration. It is therefore recommended that dosage be based upon ideal body weight for height in obese patients (see DOSAGE AND ADMINISTRATION).
Malignant Hyperthermia (MH)
Human malignant hyperthermia has not been reported with the administration of ARDUAN®. Because ARDUAN® is never used alone, and because the occurrence of malignant hyperthermia during anesthesia is possible even in the absence of known triggering agents, clinicians should be familiar with early signs, confirmatory diagnosis and treatment of malignant hyperthermia prior to the start of any anesthetic. In an animal study in MH-susceptible swine (n=7), the administration of ARDUAN® was not associated with the development of malignant hyperthermia.
Long-Term Use in the Intensive Care Unit (ICU)
No data are available on the long-term use of ARDUAN® in patients undergoing mechanical ventilation in the I.C.U.
Central Nervous System
ARDUAN® has no known effect on consciousness, the pain threshold or cerebration. Therefore, administration must be accompanied by adequate anesthesia.
Drug Interactions
ARDUAN® can be administered following recovery from succinylcholine when the latter is used to facilitate endotrachael intubation (see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY).
The use of ARDUAN® before succinylcholine, in order to attenuate some of the side effects of succinylcholine is not recommended because it has not been studied.
There are no clinical data on concomitant use of ARDUAN® and other non-depolarizing neuromuscular blocking agents.
Inhalational Anesthetics
Use of volatile inhalation anesthetics have been shown to enhance the activity of other neuromuscular blocking agents on the order of enflurane > isoflurane > halothane.
Since the neuromuscular blocking agents are routinely administered before or shortly after the administration of the inhalation anesthetic, minimal effects are generally observed on onset time and peak effect. In routine use of neuromuscular blocking agents, only clinical duration is generally affected (prolonged). No definite interaction between ARDUAN® and halothane, as used clinically, has been demonstrated. Use of isoflurane in one study of 25 patients resulted in an increase in mean clinical duration by 12%. In another study of 25 patients first anesthetized with enflurane for 5 minutes or more, the mean clinical duration was increased by 50%. Therefore, a prolonged clinical duration following initial or maintenance doses and prolonged recovery from neuromuscular blocking effect of ARDUAN® should generally be anticipated, with enflurane > isoflurane > halothane.
Antibiotics
Parenteral/intraperitoneal administration of high doses of certain antibiotics may intensify or produce neuromuscular block on their own.
The following antibiotics have been associated with various degrees of paralysis: aminoglycosides (such as neomycin, streptomycin, kanamycin, gentamicin, and dihydrostreptomycin); tetracyclines; bacitracin; polymyxin B; colistin; and sodium colistimethate. If these or other newly introduced antibiotics are used in conjunction with ARDUAN® during surgery, prolongation of neuromuscular block should be considered a possibility.
Other
Experience concerning injection of quinidine during recovery from use of other muscle relaxants suggests that recurrent paralysis may occur. This possibility must also be considered for ARDUAN®.
ARDUAN®-induced neuromuscular blockade has been counteracted by alkalosis and enhanced by acidosis in experimental animals (cat). In addition, experience with other drugs has suggested that acute (e.g., diarrhea) or chronic (e.g., adrenocortical insufficiency) electrolyte imbalance may alter neuromuscular blockade. Since electrolyte imbalance and acid-base imbalance are usually mixed, either enhancement or inhibition may occur. Magnesium salts, administered for the management of toxemia of pregnancy, may enhance neuromuscular blockade.
Drug/Laboratory Test Interactions
None known.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed to evaluate carcinogenic potential or impairment of fertility. Mutagenicity studies (Ames test, Sister Chromatid Exchange) conducted with ARDUAN® revealed no mutagenic potential.
Pregnancy Category C
A teratogenicity study has been conducted in rats using intravenously administered doses of ARDUAN® approximating the clinical dose in humans (50 µg/kg). No teratogenic effects were observed in this study. An embryotoxic effect (secondary to maternal toxicity) was observed at the highest dose administered (50 µg/kg) as demonstrated by an increase in earlier fetal resorptions. There are no adequate and well-controlled studies in pregnant women. ARDUAN® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Use in Obstetrics (Caesarean section)
There are insufficient data on placental transfer of ARDUAN® and possible related effect(s) upon the neonate following Caesarean section delivery. In addition, the duration of action of ARDUAN® exceeds the duration of operative obstetrics (Caesarean section). Therefore ARDUAN® is not recommended for use in patients undergoing C-section.
Pediatric Use
Infants (3 months to 1 year) under balanced anesthesia (2 studies in 52 infants), or halothane anesthesia (1 study in 29 infants), manifest similar dose response to ARDUAN® as do adults on a µg/kg ABW basis. Children (1 to 14 years) under balanced anesthesia (4 studies in 57 children), or halothane anesthesia (2 studies in 29 children), may be less sensitive than adults. These conclusions come from studies involving titrating patient response, by the incremental method, to approximately 1.2 times ED95. There are no data on either onset time or clinical duration of larger doses in infants or children. There are no data on maintenance dosing in infants and children.
Pharmacokinetic studies in infants and children have not been performed, therefore, no pharmacokinetic modeling of incremental dosing can be attempted. The use of ARDUAN® in neonates and infants below 3 months of age has not been investigated. Antagonism has not been systematically studied in infants or children, however, usual clinical doses of neostigmine administered following significant levels of spontaneous recovery (recovery of T1 to more than 50% of control) produced complete antagonism of residual neuromuscular block in less than 10 minutes in the majority of cases.
ADVERSE REACTIONS
The most frequent side effect of non-depolarizing blocking agents, as a class, is an extension of drug's pharmacological action beyond the time period needed for surgery and anesthesia (see CLINICAL PHARMACOLOGY). Clinical signs may vary from skeletal muscle weakness to profound and prolonged skeletal muscle paralysis resulting in respiratory insufficiency or apnea. This may be due to the drug's effect or inadequate antagonism.
The following listings are based upon U.S. clinical studies involving nearly 600 patients, utilizing a variety of premedications, varying lengths of surgical procedures and various anesthetic agents.
Adverse experiences in greater than 1% of cases and judged by the investigator to have a possible casual relationship:
Clinically significant hypotension (2.5% of cases).
Clinically significant bradycardia (1.4 % of cases).
Adverse experiences in less than 1% of cases and judged by the investigator to have a possible causal relationship:
| Cardiovascular: | hypertension, myocardial ischemia, cerebrovascular accident, thrombosis, atrial fibrillation, ventricular extrasystole. |
| Metabolic and Nutritional: | increased creatinine, hypoglycemia, hyperkalemia. |
| Musculoskeletal: | muscle atrophy, difficult intubation. |
| Nervous: | hypesthesia, CNS depression. |
| Respiratory: | dyspnea, respiratory depression, laryngismus, atelectasis. |
| Skin and Appendages: | rash, urticaria. |
| Urogenital system: | anuria. |
There have been post-marketing reports of severe allergic reactions (anaphylactic and anaphylactoid reactions) with the use of neuromuscular blocking agents of which ARDUAN® is a member. These reactions, in some cases, have been life-threatening and fatal. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (see WARNINGS and PRECAUTIONS).
OVERDOSAGE
No cases of significant accidental or intentional gross overdose have been reported. In foreign clinical studies with doses up to 200 µg/kg ABW, no non-musculoskeletal effects were seen that could be attributed to the higher dosage.
In case of relative or absolute overdosage ventilation must be supported by artificial means until no longer required. Intensified monitoring of vital organ function is required for the period of paralysis and during an extended period post recovery.
Antagonism of Neuromuscular Blockade
ANTAGONISTS (SUCH AS NEOSTIGMINE) SHOULD NOT BE ADMINISTERED PRIOR TO THE DEMONSTRATION OF SOME SPONTANEOUS RECOVERY FROM NEUROMUSCULAR BLOCKADE. THE USE OF A NERVE STIMULATOR TO DOCUMENT RECOVERY AND ANTAGONISM OF NEUROMUSCULAR BLOCKADE IS RECOMMENDED.
In an analysis (across U.S. studies) of different degrees of spontaneous recovery prior to antagonism among patients antagonized by neostigmine (usual dose 0.04 mg/kg ABW), approximately 75% of patients antagonized at a T1 of approximately 25% and approximately 42% of patients antagonized at a T1 of approximately 10% manifested a T4/T1 of 0.7 or greater within 10 minutes. When T1 had recovered to at least 10 or 25% of the preblock value, T4/ T1 was often zero; antagonism with neostigmine 0.04 mg/kg ABW was usually inadequate 10 minutes after intravenous dosing in these cases. If train-of-four monitoring is available, T4/T1 should be > zero before antagonism with neostigmine is attempted. However, if TI has recovered to at least 10% of the preblock value, additional time (more than 10 minutes) or additional neostigmine dosing usually resulted in adequate antagonism.
Patients should be evaluated for adequate clinical evidence of antagonism, e.g., 5 second head lift, adequate phonation, ventilation and upper airway maintenance. Ventilation must be supported until no longer required. As with other neuromuscular blocking agents, physicians should be alert to the possibility that the action of the drugs used to antagonize neuromuscular blockade may wear off before plasma levels of ARDUAN® (pipecuronium bromide) for injection have declined sufficiently.
Antagonism may be delayed in the presence of debilitation, carcinomatosis, and concomitant use of certain broad spectrum antibiotics, or anesthetic agents and other drugs which enhance neuromuscular blockade or separately cause respiratory depression. Under such circumstances the management is the same as that of prolonged neuromuscular blockade.
In clinical trials, edrophonium doses of 0.5 mg/kg ABW were not as effective as neostigmine doses of 0.04 mg/kg ABW in antagonizing ARDUAN®-induced neuromuscular block, and were often inadequate. Therefore, the use of edrophonium 0.5 mg/kg ABW is not recommended to antagonize ARDUAN®-induced neuromuscular blockade. The use of greater (1.0 mg/kg ABW) doses of edrophonium or of pyridostigmine has not been investigated.
DOSAGE AND ADMINISTRATION
ARDUAN® (PIPECURONIUM BROMIDE) FOR INJECTION IS FOR INTRAVENOUS USE ONLY. THIS DRUG SHOULD BE ADMINISTERED BY OR UNDER THE SUPERVISION OF EXPERIENCED CLINICIANS FAMILIAR WITH THE USE OF NEUROMUSCULAR BLOCKING AGENTS. DOSAGE MUST BE INDIVIDUALIZED IN EACH CASE.
The dosage information which follows is derived from studies based upon units of drug per unit of body weight. It is expressed in this section in units of mg/kg (instead of µg/kg) to assist the clinician in calculating individual patient dosage requirements relative to the product as supplied for clinical use. It is intended to serve as an initial guide to clinicians familiar with other neuromuscular blocking agents to acquire experience with ARDUAN®. The monitoring of twitch response is recommended to evaluate recovery from ARDUAN® and decrease the hazards of overdosage if additional doses are administered (see CLINICAL PHARMACOLOGY, and Maintenance Dosing below).
It is recommended that the clinicians administering long-acting neuromuscular blocking agents such as ARDUAN® employ a peripheral nerve stimulator to monitor drug response, need for additional relaxant and adequacy of spontaneous recovery or antagonism.
Dose for Endotracheal Intubation
The recommended initial dose of ARDUAN® (pipecuronium bromide) for injection under balanced anesthesia, halothane, isoflurane, or enflurane anesthesia in patients with normal renal function who were not obese is 0.07–0.085 mg/kg (70–85 µg/kg) (see CLINICAL PHARMACOLOGY). Good to excellent intubating conditions are generally provided within 2.5 to 3 minutes. Maximum blockade, usually > 95%, is achieved in approximately 5 minutes. Doses in this range provide approximately 1–2 hours of clinical relaxation under balanced anesthesia (range 47–124 minutes). Under halothane, isoflurane and enflurane anesthesia, extension of the period of clinical relaxation should be expected (see Inhalational Anesthetics Subsection of PRECAUTIONS).
For obese patients (30% or more above ideal body weight for height) it is particularly important that a dosage adjustment be considered and the dosage administered according to ideal body weight (see Individualization of Dosage Subsection of CLINICAL PHARMACOLOGY).
Use Following Succinylcholine
If succinylcholine is used to facilitate endotracheal intubation, ARDUAN® may be administered after recovery from succinylcholine paralysis. In patients with normal renal function who are not obese starting doses of 0.05 mg/kg (50 µg/kg) of ARDUAN® are recommended and will provide approximately 45 minutes of clinical relaxation (see CLINICAL PHARMACOLOGY). In patients with normal renal function who are not obese higher ARDUAN® doses of 0.7–0.085 mg/kg (70–85 µg/kg), if administered after recovery from succinylcholine, are associated with approximately the same clinical duration as ARDUAN® without prior administration of succinylcholine.
Maintenance Dosing
Maintenance doses of 0.010–0.015 mg/kg (10–15 µg/kg), ARDUAN® administered at 25% recovery of control T1, provide approximately 50 minutes (range 17 to 175 minutes) clinical duration under balanced anesthesia (see CLINICAL PHARMACOLOGY). A lower dose should be considered in patients receiving inhalation anesthetics (see Drug Interactions Subsection of PRECAUTIONS). In all cases, dosing should be guided based on the clinical duration following initial dose or prior maintenance dose and not administered until signs of neuromuscular function are evident
Use in Pediatrics
Infants (3 months to 1 year) under balanced anesthesia (2 studies in 52 infants), or halothane anesthesia (1 study in 20 infants), manifest similar dose response to ARDUAN® as do adults on a µg/kg ABW basis, children (1 to 14 years) under balanced anesthesia (4 studies in 57 children), or halothane anesthesia (2 studies in 29 children), may be less sensitive than adults. The clinical duration of doses averaging 0.04 mg/kg ABW (40 µg/kg) in infants, and 0.057 mg/kg ABW (57 µg/kg) in children, ranged from 10 to 44 minutes, and from 18 to 52 minutes, respectively. These doses were approximately 1.2 times ED95. There are no data on either onset time or clinical duration of larger doses used to facilitate intubation in infants or children, therefore, no specific dosage recommendations above the approximate ED95 can be made. There are no data on maintenance dosing in infants or children. The use of ARDUAN® in neonates and infants below 3 months of age has not been investigated.
Compatibility
ARDUAN® is compatible with, and can be reconstituted using the following commonly used I.V. solutions:
0.9% NaCl solution
5% glucose in saline
5% glucose in water
lactated ringer's
sterile water for injection
bacteriostatic water for injection
ARDUAN® is not recommended for dilution into and/or administration from large volume I.V. solutions.
Use within 24 hours of mixing with the above solutions.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
10 mL vials containing 10 mg lyophilized pipecuronium bromide.
Boxes of 6 NDC 0052-0446-36
Storage
2°−30°C (35°−86°F). Protect from light.
After Reconstitution
When reconstituted with bacteriostatic water for injection, USP: CONTAINS BENZYL ALCOHOL, WHICH IS NOT INTENDED FOR USE IN NEWBORNS. Use within 5 days. May be stored at room temperature or refrigerated.
When reconstituted with sterile water for injection or other compatible I.V. solutions: Refrigerate vial. Use within 24 hours. Single use only. Discard unused portion.
CAUTION: Federal law prohibits dispensing without prescription.
Manufactured by
ORGANON INC. WEST ORANGE, NEW JERSEY 07052
or by
BEN VENUE LABS., INC. BEDFORD, OHIO 44146
"®Registered Trademark: Licensed from the chemical works of
Gedeon Richtor Ltd., Budapest, Hungary"
Rev. 11/10
XXXXXXX
PRINCIPAL DISPLAY PANEL - 10 mg Label
Image not
available

| ARDUAN
pipecuronium bromide injection |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019638 | 07/26/1990 | 09/27/2001 |
| Labeler - Organon Pharmaceuticals USA (002152858) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| ORGANON INC. | 942193160 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| BEN VENUE LABS | 004327953 | MANUFACTURE | |