rituxan (rituximab) injection, solution
WARNINGS
Fatal Infusion Reactions: Deaths within 24 hours of Rituxan infusion have been reported. These fatal reactions followed an infusion reaction complex, which included hypoxia, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, or cardiogenic shock. Approximately 80% of fatal infusion reactions occurred in association with the first infusion. (See WARNINGS and ADVERSE REACTIONS.)
Patients who develop severe infusion reactions should have Rituxan infusion discontinued and receive medical treatment.
Tumor Lysis Syndrome (TLS): Acute renal failure requiring dialysis with instances of fatal outcome has been reported in the setting of TLS following treatment of non‑Hodgkin's lymphoma (NHL) patients with Rituxan. (See WARNINGS.)
Severe Mucocutaneous Reactions: Severe mucocutaneous reactions, some with fatal outcome, have been reported in association with Rituxan treatment. (See WARNINGS and ADVERSE REACTIONS.)
Progressive Multifocal Leukoencephalopathy (PML): JC virus infection resulting in PML and death has been reported in patients treated with Rituxan (See WARNINGS and ADVERSE REACTIONS.)
DESCRIPTION
The Rituxan® (Rituximab) antibody is a genetically engineered chimeric murine/human monoclonal antibody directed against the CD20 antigen found on the surface of normal and malignant B lymphocytes. The antibody is an IgG1 kappa immunoglobulin containing murine light- and heavy-chain variable region sequences and human constant region sequences. Rituximab is composed of two heavy chains of 451 amino acids and two light chains of 213 amino acids (based on cDNA analysis) and has an approximate molecular weight of 145 kD. Rituximab has a binding affinity for the CD20 antigen of approximately 8.0 nM.
The chimeric anti-CD20 antibody is produced by mammalian cell (Chinese Hamster Ovary) suspension culture in a nutrient medium containing the antibiotic gentamicin. Gentamicin is not detectable in the final product. The anti‑CD20 antibody is purified by affinity and ion exchange chromatography. The purification process includes specific viral inactivation and removal procedures. Rituximab Drug Product is manufactured from bulk Drug Substance manufactured by Genentech, Inc. (US License No. 1048).
Rituxan is a sterile, clear, colorless, preservative-free liquid concentrate for intravenous (IV) administration. Rituxan is supplied at a concentration of 10 mg/mL in either 100 mg (10 mL) or 500 mg (50 mL) single-use vials. The product is formulated for IV administration in 9 mg/mL sodium chloride, 7.35 mg/mL sodium citrate dihydrate, 0.7 mg/mL polysorbate 80, and Water for Injection. The pH is adjusted to 6.5.
CLINICAL PHARMACOLOGY
General
Rituximab binds specifically to the antigen CD20 (human B‑lymphocyte‑restricted differentiation antigen, Bp35), a hydrophobic transmembrane protein with a molecular weight of approximately 35 kD located on pre‑B and mature B lymphocytes. 1, 2 The antigen is also expressed on > 90% of B‑cell non‑Hodgkin's lymphomas (NHL), 3 but is not found on hematopoietic stem cells, pro‑B‑cells, normal plasma cells or other normal tissues. 4 CD20 regulates an early step(s) in the activation process for cell cycle initiation and differentiation, 4 and possibly functions as a calcium ion channel. 5 CD20 is not shed from the cell surface and does not internalize upon antibody binding. 6 Free CD20 antigen is not found in the circulation. 2
B‑cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis. In this setting, B‑cells may be acting at multiple sites in the autoimmune/inflammatory process, including through production of rheumatoid factor (RF) and other autoantibodies, antigen presentation, T cell activation, and/or pro‑inflammatory cytokine production. 7
Preclinical Pharmacology and Toxicology
Mechanism of Action: The Fab domain of Rituximab binds to the CD20 antigen on B lymphocytes, and the Fc domain recruits immune effector functions to mediate B‑cell lysis in vitro. Possible mechanisms of cell lysis include complement‑dependent cytotoxicity (CDC) 8 and antibody-dependent cell mediated cytotoxicity (ADCC). The antibody has been shown to induce apoptosis in the DHL‑4 human B‑cell lymphoma line. 9
Normal Tissue Cross-reactivity: Rituximab binding was observed on lymphoid cells in the thymus, the white pulp of the spleen, and a majority of B lymphocytes in peripheral blood and lymph nodes. Little or no binding was observed in the non-lymphoid tissues examined.
Pharmacokinetics
In patients with NHL given single doses at 10, 50, 100, 250 or 500 mg/m2 as an IV infusion, serum levels and the half-life of Rituximab were proportional to dose. 10 In 14 patients given 375 mg/m2 as an IV infusion for 4 weekly doses, the mean serum half-life was 76.3 hours (range, 31.5 to 152.6 hours) after the first infusion and 205.8 hours (range, 83.9 to 407.0 hours); after the fourth infusion. 11, 12, 13 The wide range of half-lives may reflect the variable tumor burden among patients and the changes in CD20‑positive (normal and malignant) B‑cell populations upon repeated administrations.
Rituxan at a dose of 375 mg/m2 was administered as an IV infusion at weekly intervals for 4 doses to 203 patients with NHL naive to Rituxan. 13, 14 The mean Cmax following the fourth infusion was 486µg/mL (range, 77.5 to 996.6 µg/mL). The peak and trough serum levels of Rituximab were inversely correlated with baseline values for the number of circulating CD20‑positive B‑cells and measures of disease burden. Median steady-state serum levels were higher for responders compared with nonresponders; however, no difference was found in the rate of elimination as measured by serum half-life. Serum levels were higher in patients with International Working Formulation (IWF) subtypes B, C, and D as compared with those with subtype A. 11, 14 Rituximab was detectable in the serum of patients 3 to 6 months after completion of treatment.
Rituxan at a dose of 375 mg/m2 was administered as an IV infusion at weekly intervals for 8 doses to 37 patients with NHL. 15 The mean Cmax after 8 infusions was 550 µg/mL (range, 171–1177 µg/mL). The mean Cmax increased with each successive infusion through the eighth infusion (Table 1).
| Infusion Number | Mean Cmax
µg/mL | Range µg/mL |
|---|---|---|
| 1 | 242.6 | 16.1–581.9 |
| 2 | 357.5 | 106.8–948.6 |
| 3 | 381.3 | 110.5–731.2 |
| 4 | 460.0 | 138.0–835.8 |
| 5 | 475.3 | 156.0–929.1 |
| 6 | 515.4 | 152.7–865.2 |
| 7 | 544.6 | 187.0–936.8 |
| 8 | 550.0 | 170.6–1177.0 |
The pharmacokinetic profile of Rituxan when administered as 6 infusions of 375 mg/m2 in combination with 6 cycles of CHOP chemotherapy was similar to that seen with Rituxan alone. 16
Following the administration of 2 doses of Rituximab in patients with rheumatoid arthritis, the mean Cmax values were 183 mcg/mL (CV=24%) for the 2 × 500 mg dose and 370 mcg/mL (CV=25%) for the 2× 1000 mg dose, respectively. Following 2× 1000 mg Rituximab dose, mean volume of distribution at steady state was 4.3 L (CV=28%). Mean systemic serum clearance of Rituximab was 0.01 L/h (CV=38%), and mean terminal elimination half‑life after the second dose was 19 days (CV=32%).
Special Populations
Gender: The female patients with RA (n=86) had a 37% lower clearance of Rituximab than male patients with RA (n=25). The gender difference in Rituximab clearance does not necessitate any dose adjustment because safety and efficacy of Rituximab do not appear to be influenced by gender.
The pharmacokinetics of Rituximab have not been studied in children and adolescents. No formal studies were conducted to examine the effects of either renal or hepatic impairment on the pharmacokinetics of Rituximab.
Pharmacodynamics
Administration of Rituxan resulted in a rapid and sustained depletion of circulating and tissue‑based B‑cells. Lymph node biopsies performed 14 days after therapy showed a decrease in the percentage of B‑cells in seven of eight patients with NHL who had received single doses of Rituximab ≥100 mg/m2. 10 Among the 166 patients in the pivotal NHL study, circulating B‑cells (measured as CD19‑positive cells) were depleted within the first three doses with sustained depletion for up to 6 to 9 months post-treatment in 83% of patients. 14 Of the responding patients assessed (n = 80), 1% failed to show significant depletion of CD19‑positive cells after the third infusion of Rituximab as compared to 19% of the nonresponding patients. B‑cell recovery began at approximately 6 months following completion of treatment. Median B‑cell levels returned to normal by 12 months following completion of treatment. 14
There were sustained and statistically significant reductions in both IgM and IgG serum levels observed from 5 through 11 months following Rituximab administration. However, only 14% of patients had reductions in IgM and/or IgG serum levels, resulting in values below the normal range. 14
In RA patients, treatment with Rituxan induced depletion of peripheral B lymphocytes, with all patients demonstrating near complete depletion within 2 weeks after receiving the first dose of Rituxan. The majority of patients showed peripheral B‑cell depletion for at least 6 months, followed by subsequent gradual recovery after that timepoint. A small proportion of patients (4%) had prolonged peripheral B‑cell depletion lasting more than 3 years after a single course of treatment.
In RA studies, total serum immunoglobulin levels, IgM, IgG, and IgA were reduced at 6 months with the greatest change observed in IgM. However, mean immunoglobulin levels remained within normal levels over the 24‑week period. Small proportions of patients experienced decreases in IgM (7%), IgG (2%), and IgA (1%) levels below the lower limit of normal. The clinical consequences of decreases in immunoglobulin levels in RA patients treated with Rituxan are unclear.
Treatment with Rituximab in patients with RA was associated with reduction of certain biologic markers of inflammation such as interleukin-6 (IL 6), C‑reactive protein (CRP), serum amyloid protein (SAA), S100 A8/S100 A9 heterodimer complex (S100 A8/9), anti‑citrullinated peptide (anti‑CCP) and RF.
CLINICAL STUDIES
Relapsed or Refractory, Low‑Grade or Follicular, CD‑20 Positive, B‑Cell, NHL
Rituxan regimens tested include treatment weekly for 4 doses and treatment weekly for 8 doses. Results for studies with a collective enrollment of 296 patients are summarized below (Table 2):
| Study 1 Weekly× 4 N = 166 | Study 2 Weekly× 8 N = 37 | Study 1 and Study 3 Bulky disease, Weekly× 4 N = 39* | Study 3 Retreatment, Weekly × 4 N = 60 |
|
|---|---|---|---|---|
| Overall Response Rate | 48% | 57% | 36% | 38% |
| Complete Response Rate | 6% | 14% | 3% | 10% |
| Median Duration Of
Response†,‡,§
(Months) [Range] | 11.2 [1.9 to 42.1+] | 13.4 [2.5 to 36.5+] | 6.9 [2.8 to 25.0+] | 15.0 [3.0 to 25.1+] |
Weekly for 4 Doses
Study 1
A multicenter, open‑label, single‑arm study was conducted in 166 patients with relapsed or refractory, low‑grade or follicular B‑cell NHL who received 375 mg/m2 of Rituxan given as an IV infusion weekly for 4 doses. 14 Patients with tumor masses>10 cm or with >5000 lymphocytes/µL in the peripheral blood were excluded from the study. Results are summarized in Table 2. The median time to onset of response was 50 days and the median duration of response was 11.2 months (range, 1.9–42.1+). Disease‑related signs and symptoms (including B‑symptoms) were present in 23% (39/166) of patients at study entry and resolved in 64% (25/39) of those patients.
In a multivariate analysis, the ORR was higher in patients with IWF B, C, and D histologic subtypes as compared to IWF subtype A (58% vs. 12%), higher in patients whose largest lesion was<5 cm vs. >7 cm (maximum, 21 cm) in greatest diameter (53% vs. 38%), and higher in patients with chemosensitive relapse as compared with chemoresistant (defined as duration of response<3 months) relapse (53% vs. 36%). ORR in patients previously treated with autologous bone marrow transplant was 78% (18/23). The following adverse prognostic factors were not associated with a lower response rate: age ≥60 years, extranodal disease, prior anthracycline therapy, and bone marrow involvement.
Weekly for 8 Doses
Study 2
In a multicenter, single‑arm study, 37 patients with relapsed or refractory, low‑grade NHL received 375 mg/m2 of Rituxan weekly for 8 doses. Results are summarized in Table 2. (see ADVERSE REACTIONS: Risk Factors Associated with Increased Rates of Adverse Events.)
Bulky Disease, Weekly for 4 Doses
In pooled data (Study 1 and 3) from multiple studies of Rituxan, 39 patients with relapsed or refractory, bulky disease (single lesion>10 cm in diameter), low-grade NHL received 375 mg/m2 of Rituxan weekly for 4 doses. Results are summarized in Table 2. 16, 17 (For information on the higher incidence of Grade 3 and 4 adverse events, see ADVERSE REACTIONS: Risk Factors Associated with Increased Rates of Adverse Events.)
Retreatment Weekly for 4 Doses
Study 3
In a multicenter, single‑arm study, 60 patients received 375 mg/m2 of Rituxan weekly for 4 doses. 18 All patients had relapsed or refractory, low‑grade or follicular B‑cell NHL and had achieved an objective clinical response to Rituxan administered 3.8–35.6 months (median 14.5 months) prior to retreatment with Rituxan. Of these 60 patients, 55 received their second course of Rituxan, 3 patients received their third course and 2 patients received their second and third courses of Rituxan in this study. Results are summarized in Table 2.
Previously Untreated, Follicular, CD‑20 Positive, B‑Cell NHL
Study 4
A total of 322 patients with previously untreated follicular NHL were randomized (1:1) to receive up to eight 3‑week cycles of CVP chemotherapy alone (CVP) or in combination with Rituxan 375 mg/m2 on Day 1 of each cycle (R‑CVP) in an open‑label, multicenter study. The main outcome measure of the study was progression‑free survival (PFS) defined as the time from randomization to the first of progression, relapse or death.
Twenty‑six percent of the study population was >60 years of age, 99% had Stage III or IV disease, and 50% had an International Prognostic Index (IPI) score ≥2. Of the 289 patients with available histologic material for review, 95% had a centrally‑confirmed diagnosis of follicular (REAL follicular grade 1, 2 and 3) NHL. The results for PFS as determined by a blinded, independent assessment of progression are presented in Table 3. The point estimates may be influenced by the presence of informative censoring. The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
| Study Arm | ||
|---|---|---|
| CVP | R‑CVP | |
| Median PFS (years)* | 1.4 | 2.4 |
| Hazard ratio (95% CI)† | 0.44 (0.29, 0.65) | |
Previously Untreated, Low Grade, CD‑20 Positive, B‑Cell NHL
Study 5
A total of 322 patients with previously untreated low‑grade, B‑cell NHL (IWF Grades A, B or C) who did not progress after 6 or 8 cycles of CVP chemotherapy were enrolled in an open-label, multicenter, randomized trial. Patients were randomized (1:1) to receive Rituxan, 375 mg/m2 IV infusion, once weekly for 4 doses every 6 months for up to 16 doses or no further therapeutic intervention. The main outcome measure of the study was progression‑free survival defined as the time from randomization to progression, relapse or death. Thirty‑seven percent of the study population was >60 years of age, 99% had Stage III or IV disease, and 63% had an IPI score ≥2. Among the 237 patients for whom histologic material was available for review, 201 patients (85%) had centrally confirmed IWF Grade A, B or C NHL.
There was a reduction in the risk of progression, relapse, or death (hazard ratio estimate in the range of 0.36 to 0.49) for patients randomized to Rituxan as compared to those who received no additional treatment.
Diffuse Large B‑Cell NHL (DLBCL)
The safety and effectiveness of Rituxan were evaluated in three, randomized, active‑controlled, open‑label, multicenter studies with a collective enrollment of 1854 patients. Patients with previously untreated diffuse large B‑cell NHL received Rituxan in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or other anthracycline‑based chemotherapy regimens.
Study 6
A total of 632 patients aged≥60 years with either B‑cell NHL Grade F, G, or H by the International Working Formulation classification or DLBCL (including primary mediastinal B‑cell lymphoma) in the REAL classification were randomized in a 1:1 ratio to treatment with CHOP or R‑CHOP. Patients were given 6 or 8, 21 day cycles of CHOP. Patients in the R‑CHOP arm also received 4 or 5 doses of Rituxan 375 mg/m2 on Days−7 and −3 (prior to Cycle 1), and 48–72 hours pre–Cycle 3, pre–Cycle 5, and pre–Cycle 7 for patients receiving 8 cycles of CHOP induction. The main outcome measure of the study was progression–free survival, defined as the time from randomization to the first of progression, relapse or death. Responding patients underwent a second randomization to receive Rituxan or no further therapy.
Among all enrolled patients, 62% had centrally confirmed DLBCL histology, 73% had Stage III–IV disease, 56% had IPI scores ≥2, 86% had ECOG performance status of <2, 57% had elevated LDH levels, and 30% had two or more extranodal disease sites involved. Efficacy results are presented in Table 4. These results reflect a statistical approach which allows for an evaluation of Rituxan administered in the induction setting that excludes any potential impact of Rituxan given after the second randomization.
Analysis of results after the second randomization in Study 6 demonstrates that for patients randomized to R‑CHOP, additional Rituxan exposure beyond induction was not associated with further improvements in progression free survival or overall survival.
Study 7
A total of 399 patients with DLBCL, aged≥60 years, were randomized in a 1:1 ratio to receive CHOP or R‑CHOP induction. All patients received up to 8, 3–week cycles of CHOP induction; patients in the R‑CHOP arm received Rituxan 375 mg/m2 on Day 1 of each cycle. The main outcome measure of the study was event free survival, defined as the time from randomization to relapse, progression, change in therapy or death from any cause. Among all enrolled patients, 80% had stage III or IV disease, 60% of patients had an age-adjusted IPI≥2, 80% had ECOG performance status scores <2, 66% had elevated LDH levels, and 52% had extranodal involvement in at least two sites. Efficacy results are presented in Table 4.
Study 8
A total of 823 patients with DLBCL, aged 18–60 years, were randomized in a 1:1 ratio to receive an anthracycline-containing chemotherapy regimen alone or in combination with Rituxan. The main outcome measure of the study was time to treatment failure, defined as time from randomization to the earliest of progressive disease, failure to achieve a complete response, relapse or death. Among all enrolled patients, 28% had Stage III–IV disease, 100% had IPI scores of≤1, 99% had ECOG performance status of<2, 29% had elevated LDH levels, 49% had bulky disease and 34% had extranodal involvement. Efficacy results are presented in Table 4.
| Study
6 (n=632) _________________________ | Study
7 (n=399) _________________________ | Study
8 (n=823) _________________________ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CHOP | R‑CHOP | CHOP | R‑CHOP | Chemo | R‑Chemo | ||||
| Main outcome | Progression‑free
survival (years) | Event‑free
survival (years) | Time
to treatment
failure (years) |
||||||
| Median of main outcome measure | 1.6 | 3.1 | 1.1 | 2.9 | NE* | NE* | |||
| Hazard ratio† | 0.69‡ | 0.60‡ | 0.45‡ | ||||||
| Overall survival at 2 years§ | 63% | 74% | 58% | 69% | 86% | 95% | |||
| Hazard ratio† | 0.72‡ | 0.68‡ | 0.40‡ | ||||||
In Study 7, overall survival estimates at 5 years were 58% vs. 46% for R‑CHOP and CHOP, respectively.
Rheumatoid Arthritis (RA)
The efficacy and safety of Rituxan were evaluated in 517 patients with active disease who were receiving methotrexate and had a prior inadequate response to at least one TNF inhibitor. Patients were ≥ 18 years, diagnosed with RA according to American College of Rheumatology (ACR) criteria and had at least 8 swollen and 8 tender joints. Patients received 2 doses of either Rituxan 1000 mg or placebo as an IV infusion on days 1 and 15, in combination with continued methotrexate 10‑25 mg weekly.
Efficacy was assessed at 24 weeks. Glucocorticoids were given IV as premedication prior to each Rituxan infusion and orally on a tapering schedule from baseline through Day 16.
The proportions of Rituxan (1000 mg) treated patients achieving ACR 20, 50, and 70 responses in this study is shown in Table 5.
| Response | Placebo + MTX n = 201 | Rituxan + MTX n = 298 |
|---|---|---|
| ACR 20 | 18% | 51% p<0.0001 |
| ACR 50 | 5% | 27% p<0.0001 |
| ACR 70 | 1% | 12% p<0.0001 |
Improvement was also noted for all components of ACR response following treatment with Rituxan, as shown in Table 6.
| Placebo + MTX (n = 201) | Rituxan + MTX (n = 298) |
|||
|---|---|---|---|---|
| Parameter (median) | Baseline | Wk 24 | Baseline | Wk 24 |
| Tender Joint Count | 31.0 | 27.0 | 33.0 | 13.0* |
| Swollen Joint Count | 20.0 | 19.0 | 21.0 | 9.5* |
| Physician Global Assessment† | 71.0 | 69.0 | 71.0 | 36.0* |
| Patient Global Assessment† | 73.0 | 68.0 | 71.0 | 41.0* |
| Pain† | 68.0 | 68.0 | 67.0 | 38.5* |
| Disability Index (HAQ)‡ | 2.0 | 1.9 | 1.9 | 1.5* |
| CRP (mg/dL) | 2.4 | 2.5 | 2.6 | 0.9* |
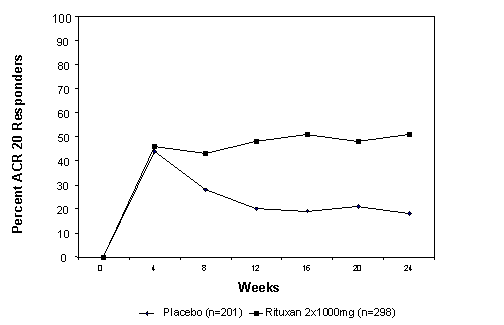
The time course of ACR 20 response for this study is shown in Figure 1. Although both treatment groups received a brief course of IV and oral glucocorticoids, resulting in similar benefits at week 4, higher ACR 20 responses were observed for the Rituxan group by week 8 and were maintained through week 24 after a single course of treatment (2 infusions) with Rituxan. Similar patterns were demonstrated for ACR 50 and 70 responses.
 |
While the efficacy of Rituxan was supported by two well‑controlled trials in RA patients who had inadequate responses to non‑biologic DMARDs, but who had not failed TNF antagonist therapy, a favorable risk benefit relationship has not been established in this population (See PRECAUTIONS.)
INDICATIONS AND USAGE
Non‑Hodgkin's Lymphoma
Rituxan® (Rituximab) is indicated for the treatment of patients with relapsed or refractory, low‑grade or follicular, CD20‑positive, B‑cell, non‑Hodgkin's lymphoma.
Rituxan® (Rituximab) is indicated for the first‑line treatment of follicular, CD20‑positive, B‑cell non‑Hodgkin’s lymphoma in combination with CVP chemotherapy.
Rituxan® (Rituximab) is indicated for the treatment of low‑grade, CD20‑positive, B‑cell non‑Hodgkin’s lymphoma in patients with stable disease or who achieve a partial or complete response following first‑line treatment with CVP chemotherapy.
Rituxan® (Rituximab) is indicated for the first–line treatment of diffuse large B‑cell, CD20‑positive, non‑Hodgkin’s lymphoma in combination with CHOP or other anthracycline‑based chemotherapy regimens.
Rheumatoid Arthritis
Rituxan® (Rituximab) in combination with methotrexate is indicated to reduce signs and symptoms in adult patients with moderately- to severely- active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies.
CONTRAINDICATIONS
None.
WARNINGS
(See BOXED WARNINGS)
Severe Infusion Reactions
(see BOXED WARNINGS and ADVERSE REACTIONS)
Rituxan has caused severe infusion reactions. In some cases, these reactions were fatal. These severe reactions typically occurred during the first infusion with time to onset of 30–120 minutes. Signs and symptoms of severe infusion reactions may include urticaria, hypotension, angioedema, hypoxia, or bronchospasm, and may require interruption of Rituxan administration. The most severe manifestations and sequelae include pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, cardiogenic shock, and anaphylactic and anaphylactoid events. In the reported cases, the following factors were more frequently associated with fatal outcomes: female gender, pulmonary infiltrates, and chronic lymphocytic leukemia or mantle cell lymphoma.
Management of severe infusion reactions: The Rituxan infusion should be interrupted for severe reactions. Medications and supportive care measures including, but not limited to, epinephrine, antihistamines, glucocortisoids, intravenous fluids, vasopressors, oxygen, bronchodilators, and acetaminophen, should be available for immediate use and instituted as medically indicated for use in the event of a reaction during administration. In most cases, the infusion can be resumed at a 50% reduction in rate (e.g., from 100 mg/hr to 50 mg/hr) when symptoms have completely resolved. Patients requiring close monitoring during first and all subsequent infusions include those with pre-existing cardiac and pulmonary conditions, those with prior clinically significant cardiopulmonary adverse events and those with high numbers of circulating malignant cells (≥ 25,000/mm3) with or without evidence of high tumor burden. (See WARNINGS: Cardiovascular and ADVERSE REACTIONS.)
Tumor Lysis Syndrome [TLS]
(See BOXED WARNINGS and ADVERSE REACTIONS)
Rapid reduction in tumor volume followed by acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, or hyperphosphatasemia, have been reported within 12–24 hours after the first Rituxan infusion. Rare instances of fatal outcome have been reported in the setting of TLS following treatment with Rituxan in patients with NHL. The risks of TLS appear to be greater in patients with high numbers of circulating malignant cells (≥ 25,000/mm3) or high tumor burden. Prophylaxis for TLS should be considered for patients at high risk. Correction of electrolyte abnormalities, monitoring of renal function and fluid balance, and administration of supportive care, including dialysis, should be initiated as indicated. Following complete resolution of the complications of TLS, Rituxan has been tolerated when re-administered in conjunction with prophylactic therapy for TLS in a limited number of cases.
Hepatitis B Reactivation with Related Fulminant Hepatitis and Other Viral Infections
Hepatitis B virus (HBV) reactivation with fulminant hepatitis, hepatic failure, and death has been reported in patients with hematologic malignancies treated with Rituxan. The majority of patients received Rituxan in combination with chemotherapy. The median time to the diagnosis of hepatitis was approximately 4 months after the initiation of Rituxan and approximately one month after the last dose.
Persons at high risk of HBV infection should be screened before initiation of Rituxan. Carriers of hepatitis B should be closely monitored for clinical and laboratory signs of active HBV infection and for signs of hepatitis during and for up to several months following Rituxan therapy. In patients who develop viral hepatitis, Rituxan and any concomitant chemotherapy should be discontinued and appropriate treatment including antiviral therapy initiated. There are insufficient data regarding the safety of resuming Rituxan therapy in patients who develop hepatitis subsequent to HBV reactivation.
The following additional serious viral infections, either new, reactivated or exacerbated, have been identified in clinical studies or postmarketing reports. The majority of patients received Rituxan in combination with chemotherapy or as part of a hematopoietic stem cell transplant. These viral infections included cytomegalovirus, herpes simplex virus, parvovirus B19, varicella zoster virus, West Nile virus, and hepatitis C. In some cases, the viral infections occurred up to one year following discontinuation of Rituxan and have resulted in death.
Progressive Multifocal Leukoencephalopathy (PML)
(See BOXED WARNINGS and ADVERSE REACTIONS)
JC virus infection resulting in PML and death has been reported in Rituxan‑treated patients with hematologic malignancies or with systemic lupus erythematosus (SLE), an indication for which Rituxan has not been approved. The majority of patients with hematologic malignancies diagnosed with PML have received Rituxan in combination with chemotherapy or as part of a hematopoietic stem cell transplant. Patients with SLE had a history of prior immunosuppressive therapy and were diagnosed with PML within 12 months of their last infusion of Rituxan.
Physicians treating patients with Rituxan should consider PML in any patient presenting with new onset neurologic manifestations. Consultation with a neurologist, brain MRI, and lumbar puncture should be considered as clinically indicated. In patients who develop PML, Rituxan should be discontinued and reductions or discontinuation of any concomitant chemotherapy or immunosuppressive therapy should be considered.
Cardiovascular
Infusions should be discontinued in the event of serious or life-threatening cardiac arrhythmias. Patients who develop clinically significant arrhythmias should undergo cardiac monitoring during and after subsequent infusions of Rituxan Patients with pre-existing cardiac conditions including arrhythmias and angina have had recurrences of these events during Rituxan therapy and should be monitored throughout the infusion and immediate post-infusion period.
Renal
(See BOXED WARNINGS: Tumor Lysis Syndrome [TLS] and ADVERSE REACTIONS)
Rituxan administration has been associated with severe renal toxicity including acute renal failure requiring dialysis and in some cases, has led to a fatal outcome in hematologic malignancy patients. Renal toxicity has occurred in patients with high numbers of circulating malignant cells (>25,000/mm3 ) or high tumor burden who experience tumor lysis syndrome and in patients with NHL administered concomitant cisplatin therapy during clinical trials. The combination of cisplatin and Rituxan is not an approved treatment regimen. If this combination is used in clinical trials extreme caution should be exercised; patients should be monitored closely for signs of renal failure. Discontinuation of Rituxan should be considered for those with rising serum creatinine or oliguria.
Severe Mucocutaneous Reactions
(See BOXED WARNINGS)
Mucocutaneous reactions, some with fatal outcome, have been reported in patients treated with Rituxan. These reports include paraneoplastic pemphigus (an uncommon disorder which is a manifestation of the patient's underlying malignancy), 19 Stevens‑Johnson syndrome, lichenoid dermatitis, vesiculobullous dermatitis, and toxic epidermal necrolysis. The onset of the reaction in the reported cases has varied from 1–13 weeks following Rituxan exposure. Patients experiencing a severe mucocutaneous reaction should not receive any further infusions and seek prompt medical evaluation. Skin biopsy may help to distinguish among different mucocutaneous reactions and guide subsequent treatment. The safety of readministration of Rituxan to patients with any of these mucocutaneous reactions has not been determined.
Concomitant use with biologic agents and DMARDs other than methotrexate in RA: Limited data are available on the safety of the use of biologic agents or DMARDs other than methotrexate in patients exhibiting peripheral B cell depletion following treatment with Rituximab. Patients should be closely observed for signs of infection if biologic agents and/or DMARDs are used concomitantly.
Bowel Obstruction and Perforation
Abdominal pain, bowel obstruction and perforation, in some cases leading to death, were observed in patients receiving Rituxan in combination with chemotherapy for DLBCL. In post‑marketing reports, which include both patients with low‑grade or follicular NHL and DLBCL, the mean time to onset of symptoms was 6 days (range 1–77) in patients with documented gastro‑intestinal perforation. Complaints of abdominal pain, especially early in the course of treatment, should prompt a thorough diagnostic evaluation and appropriate treatment.
PRECAUTIONS
Information for Patients
Patients should be provided the Rituxan Patient Information leaflet and provided an opportunity to read it prior to each treatment session. Because caution should be exercised in administering Rituxan to patients with active infections, it is important that the patient’s overall health be assessed at each visit and any questions resulting from the patient’s reading of the Patient Information be discussed.
Laboratory Monitoring
Because Rituxan targets all CD20-positive B lymphocytes, (malignant and nonmalignant), complete blood counts (CBC) and platelet counts should be obtained at regular intervals during Rituxan therapy and more frequently in patients who develop cytopenias (see ADVERSE REACTIONS). The duration of cytopenias caused by Rituxan can extend well beyond the treatment period.
Drug/Laboratory Interactions
There have been no formal drug interaction studies performed with Rituxan. However, renal toxicity was seen with this drug in combination with cisplatin in clinical trials. (See WARNINGS: Renal.) In clinical trials of patients with RA, concomitant administration of methotrexate or cyclophosphamide did not alter the pharmacokinetics of Rituximab.
Immunization
The safety of immunization with live viral vaccines following Rituxan therapy has not been studied and vaccination with live virus vaccines is not recommended. The ability to generate a primary or anamnestic humoral response to vaccination is currently being studied.
Physicians should review the vaccination status of patients with RA being considered for Rituxan treatment and follow the Centers for Disease Control and Prevention (CDC) guidelines for adult vaccination with non-live vaccines untended to prevent infectious disease, prior to therapy. For patients with NHL, the benefits of primary and/or booster vaccinations should be weighted against the risks of delay in initiation of Rituxan therapy.
Use in patients with RA who had no prior inadequate response to TNF antagonists: While efficacy of Rituxan was supported in two well-controlled trials in patients with RA with prior inadequate responses to non-biologic DMARDs, a favorable risk benefit relationship has not been established in this population. The use of Rituxan in patients with RA who have no prior inadequate response to one or more TNF antagonists is not recommended (see CLINICAL STUDIES: Rheumatoid Arthritis).
Retreatment in patients with RA: Safety and efficacy of retreatment have not been established in controlled trials. A limited number of patients have received two to five courses (two infusions per course) of treatment in an uncontrolled setting. In clinical trials in patients with RA, most of the patients who received additional courses did so 24 weeks after the previous course and none were retreated sooner than 16 weeks.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No long-term animal studies have been performed to establish the carcinogenic potential of Rituxan. Studies also have not been completed to assess mutagenic potential of Rituxan, or to determine potential effects on fertility in males or females. Individuals of childbearing potential should use effective contraceptive methods during treatment and for up to 12 months following Rituxan therapy.
Pregnancy Category C
An embryo-fetal developmental toxicity study was performed on pregnant cynomolgus monkeys. Animals were administered Rituximab via the intravenous route during early gestation (organogenesis period; post‑coitum days 20 through 50). Rituximab was administered as loading doses on post-coitum days 20, 21 and 22, at 15, 37.5 or 75 mg/kg/day, and then weekly on post-coitum days 29, 36, 43 and 50, at 20, 50 or 100 mg/kg/week. The 100 mg/kg/week dose resulted in exposures of 0.8‑fold a human 2 g dose based on AUC. Although Rituximab has been shown to cross the monkey placenta, there was no evidence of teratogenicity under the conditions of the experiment.
Nonteratogenic effects: Results from the embryo‑fetal developmental toxicology study described above showed that Rituximab treatment produced a decrease in lymphoid tissue B cells in the offspring of treated dams.
A subsequent pre- and postnatal developmental toxicity study in cynomolgus monkeys was completed to assess developmental toxicity and the recovery of B‑cells and immune function in infants exposed to Rituximab in utero. Rituximab was administered from early gestation (post‑coitum day 20) through lactation (post‑partum day 28). Due to the possibility of anti-drug antibody development with such a long dosing period, the animals were divided into 3 sets of dosing periods: one set received Rituximab (20 or 100 mg/kg weekly) from post‑coitum day 20 through delivery and post-partum day 28 (~25 weeks); a second set received Rituximab (20 or 100 mg/kg weekly) from post‑coitum day 50 through post‑coitum day 76 (8 weeks); a third set received Rituximab (20 or 100 mg/kg weekly) from post‑coitum day 76 through delivery and post‑partum day 28 (~8 weeks). For each of these dosing periods, a loading dose was administered for the first 3 days of the period at doses of 15 or 75 mg/kg/day. The decreased B cells and immunosuppression noted in the offspring of pregnant animals treated with either 20 or 100 mg/kg/week Rituximab showed a return to normal levels and function within 6 months post‑birth. However, there are no adequate and well‑controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Rituximab was excreted in the milk of lactating cynomolgus monkeys. It is not known whether Rituxan is excreted in human milk. Because human IgG is excreted in human milk and the potential for absorption and immunosuppression in the infant is unknown, women should be advised to discontinue nursing until circulating drug levels are no longer detectable. (See CLINICAL PHARMACOLOGY.)
Pediatric Use
The safety and effectiveness of Rituxan in pediatric patients have not been established.
Geriatric Use
Among patients with DLBCL in three randomized, active-controlled trials, 927 patients received Rituxan in combination with chemotherapy. Of these, 396 (43%) were age 65 or greater and 123 (13%) were age 75 or greater. No overall differences in effectiveness were observed between these subjects and younger subjects. However, elderly patients were more likely to experience cardiac adverse events, mostly supraventricular arrhythmias. Serious pulmonary adverse events were also more common among the elderly, including pneumonia and pneumonitis.
Clinical studies of Rituxan in previously untreated, low‑grade or follicular, CD‑20 positive, B‑cell NHL and in relapsed or refractory, low‑grade or follicular lymphoma did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Among the 517 patients in the phase 3 RA study, 16% were 65–75 years old and 2% were 75 years old and older. The Rituxan ACR 20 response rates in the older (age ≥65 years) vs. younger (age<65 years) patients were similar (53% vs. 51%, respectively). Adverse reactions, including incidence, severity, and type of adverse reaction were similar between older and younger patients.
ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
The following serious adverse reactions, some with fatal outcomes, have been reported in patients treated with Rituxan (see BOXED WARNINGS and WARNINGS): severe or fatal infusion reactions, tumor lysis syndrome, severe mucocutaneous reactions, hepatitis B reactivation with fulminant hepatitis, progressive multifocal leukoencephalopathy (PML), other viral infections, cardiac arrhythmias, renal toxicity, bowel obstruction and perforation.
Adverse Reactions in Patients with Non-Hodgkin's Lymphoma
The overall safety database for Rituxan is based on clinical trial data from 1606 patients with NHL, who received Rituxan either as a single agent or in combination with chemotherapy. Additional safety information was obtained from post-marketing safety surveillance. The most common adverse reactions were infusion reactions (see INFUSION REACTIONS below).
Except as noted, adverse events described below occurred in the setting of relapsed or refractory, low–grade or follicular, CD20-positive, B‑cell, NHL and are based on 356 patients treated in single‑arm studies of Rituxan administered as a single agent. Most patients received Rituxan 375 mg/m2 weekly for 4 doses.
Infusion Reactions
(See BOXED WARNINGS and WARNINGS)
Mild to moderate infusion reactions consisting of fever and chills/rigors occurred in the majority of patients during the first Rituxan infusion. Other frequent infusion reaction symptoms included nausea, pruritus, angioedema, asthenia, hypotension, headache, bronchospasm, throat irritation, rhinitis, urticaria, rash, vomiting, myalgia, dizziness, and hypertension. These reactions generally occurred within 30 to 120 minutes of beginning the first infusion, and resolved with slowing or interruption of the Rituxan infusion and with supportive care (diphenhydramine, acetaminophen, IV saline, and vasopressors). The incidence of infusion reactions was highest during the first infusion (77%) and decreased with each subsequent infusion (30% with fourth infusion and 14% with eighth infusion). Injection site pain was reported in less than 5% of patients.
Infectious Events
(See WARNINGS: Hepatitis B Reactivation with Related Fulminant Hepatitis and Other Viral Infections; Progressive Multifocal Leukoencephalopathy (PML))
Rituxan induced B‑cell depletion in 70% to 80% of patients with NHL and was associated with decreased serum immunoglobulins in a minority of patients; the lymphopenia lasted a median of 14 days (range, 1–588 days). Infectious events occurred in 31% of patients: 19% of patients had bacterial infections, 10% had viral infections, 1% had fungal infections, and 6% were unknown infections. Incidence is not additive because a single patient may have had more than one type of infection. Serious infectious events (Grade 3 or 4), including sepsis, occurred in 2% of patients.
Hematologic Events
Grade 3 and 4 cytopenias were reported in 48% of patients treated with Rituxan; these include: lymphopenia (40%), neutropenia (6%), leukopenia (4%), anemia (3%), and thrombocytopenia (2%). The median duration of lymphopenia was 14 days (range, 1–588 days) and of neutropenia was 13 days (range, 2–116 days). A single occurrence of transient aplastic anemia (pure red cell aplasia) and two occurrences of hemolytic anemia following Rituxan therapy were reported.
Pulmonary Events
135 patients (38%) experienced pulmonary events in clinical trials. The most common respiratory system adverse events experienced were increased cough, rhinitis, bronchospasm, dyspnea, and sinusitis. In both clinical studies and post-marketing surveillance, there have been a limited number of reports of bronchiolitis obliterans presenting up to 6 months post-Rituxan infusion and a limited number of reports of pneumonitis (including interstitial pneumonitis) presenting up to 3 months post-Rituxan infusion, some of which resulted in fatal outcomes. The safety of resumption or continued administration of Rituxan in patients with pneumonitis or bronchiolitis obliterans is unknown.
Immunogenicity
The observed incidence of antibody positivity in an assay is highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors including sample handling, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Rituxan with the incidence of antibodies to other products may be misleading.
In clinical studies of patients with low–grade or follicular NHL receiving single–agent Rituxan, human antichimeric antibody (HACA) was detected in 4 of 356 (1.1%) patients and 3 had an objective clinical response. These data reflect the percentage of patients whose test results were considered positive for antibodies to Rituxan using an enzyme-linked immunosorbant assay (limit of detection = 7 ng/mL).
Single Agent Rituxan for Relapsed or Refractory, Low‑Grade or Follicular, CD20‑Positive, B‑Cell NHL
The data below were obtained in 356 patients receiving single agent Rituxan for treatment of relapsed, refractory, low grade or follicular NHL (see CLINICAL STUDIES). The majority of patients received 375 mg/m2 IV weekly × 4 doses. The median age was 57 (range 22–81 years). Sixty percent were male; 93% were Caucasian, 1% were Black, 2% were Hispanic, 2% were Asian, and 2% were from other racial groups.
Table 7 lists the most common, as well as Grade 3 and 4, adverse events observed.
| All Grades (%) | Grade 3 and 4 (%) | |
|---|---|---|
| Any Adverse Events | 99 | 57 |
| Body as a Whole | 86 | 10 |
| Fever | 53 | 1 |
| Chills | 33 | 3 |
| Infection | 31 | 4 |
| Asthenia | 26 | 1 |
| Headache | 19 | 1 |
| Abdominal Pain | 14 | 1 |
| Pain | 12 | 1 |
| Back Pain | 10 | 1 |
| Throat Irritation | 9 | 0 |
| Flushing | 5 | 0 |
| Cardiovascular System | 25 | 3 |
| Hypotension | 10 | 1 |
| Hypertension | 6 | 1 |
| Digestive System | 37 | 2 |
| Nausea | 23 | 1 |
| Diarrhea | 10 | 1 |
| Vomiting | 10 | 1 |
| Hemic and Lymphatic System | 67 | 48 |
| Lymphopenia | 48 | 40 |
| Leukopenia | 14 | 4 |
| Neutropenia | 14 | 6 |
| Thrombocytopenia | 12 | 2 |
| Anemia | 8 | 3 |
| Metabolic and Nutritional Disorders | 38 | 3 |
| Angioedema | 11 | 1 |
| Hyperglycemia | 9 | 1 |
| Peripheral Edema | 8 | 0 |
| LDH Increase | 7 | 0 |
| Musculoskeletal System | 26 | 3 |
| Myalgia | 10 | 1 |
| Arthralgia | 10 | 1 |
| Nervous System | 32 | 1 |
| Dizziness | 10 | 1 |
| Anxiety | 5 | 1 |
| Respiratory System | 38 | 4 |
| Increased Cough | 13 | 1 |
| Rhinitis | 12 | 1 |
| Bronchospasm | 8 | 1 |
| Dyspnea | 7 | 1 |
| Sinusitis | 6 | 0 |
| Skin and Appendages | 44 | 2 |
| Night Sweats | 15 | 1 |
| Rash | 15 | 1 |
| Pruritus | 14 | 1 |
| Urticaria | 8 | 1 |
Risk Factors Associated with Increased Rates of Adverse Events
Administration of Rituxan weekly for 8 doses resulted in higher rates of Grade 3 and 4 adverse events 15 overall (70%) compared with administration weekly for 4 doses (57%). The incidence of Grade 3 or 4 adverse events was similar in patients retreated with Rituxan compared with initial treatment (58% and 57%, respectively). The incidence of the following clinically significant adverse events was higher in patients with bulky disease (lesions ≥10 cm) (N = 39) versus patients with lesions< 10 cm (N = 195): abdominal pain, anemia, dyspnea, hypotension, and neutropenia.
Previously Untreated, Follicular, CD20‑Positive, B‑Cell NHL
The safety data were obtained in a single, multi‑center, randomized study of 321 patients of whom 162 received Rituxan in combination with CVP chemotherapy (R‑CVP) and 159 received CVP chemotherapy alone (CVP). Eighty‑five percent of R‑CVP patients received the maximum number of doses (8) of Rituxan. The median age was 52 years, 54% were male, and 96% were Caucasian.
Patients in the R‑CVP arm had higher incidences of infusional toxicity and of neutropenia as compared to those in the CVP arm. The following adverse events occurred more frequently (≥5%) in patients receiving R‑CVP compared to CVP alone: rash (17% vs. 5%), cough (15% vs. 6%), flushing (14% vs. 3%), rigors (10% vs. 2%), pruritis (10% vs. 1%), neutropenia (8% vs. 3%), and chest tightness (7% vs. 1%).
Previously Untreated, Low‑Grade, CD20‑Positive, B‑Cell NHL
Safety data were obtained in a single, multi‑center, randomized study of 322 patients of whom 161 received Rituxan and 161 received no treatment following 6–8 cycles of CVP chemotherapy. Ninety‑five patients (59%) received the maximum number of doses (16) of Rituxan.
The median age for the Rituxan treated patients was 58 years. Fifty‑five percent were male, 93% were Caucasian, and 5% Black.
The following adverse events were reported more frequently (≥5%) in patients receiving Rituxan following CVP compared with those who received no further therapy: fatigue (39% vs. 14%), anemia (35% vs. 20%), peripheral sensory neuropathy (30% vs. 18%), infections (19% vs. 9%), pulmonary toxicity (18% vs. 10%), hepato biliary toxicity (17% vs. 7%), rash and/or pruritis (17% vs. 5%), arthralgia (12% vs. 3%), and weight gain (11% vs. 4%). Neutropenia was the only Grade 3 or 4 adverse event that occurred more frequently (≥2%) in the Rituxan arm compared with those who received no further therapy (4% vs. 1%).
Rituxan in Combination with Chemotherapy for DLBCL
Adverse events described in the setting of DLBCL are based on three randomized, active-controlled clinical trials in which 927 patients received Rituxan in combination with chemotherapy and 802 patients received chemotherapy alone. Detailed safety data collection was primarily limited to Grade 3 and 4 adverse events and serious adverse events.
The population varied from 18–92 years of age and 55% were male; racial distribution was collected only for Study 6 (see CLINICAL STUDIES section) where 90% of patients were Caucasian, 5% were Black, 3% were Hispanic and 2% were from other racial groups. Patients received 4–8 doses of Rituxan at 375 mg/m2.
The following adverse events, regardless of severity, were reported more frequently ( ≥5%) in patients age ≥60 years receiving R‑CHOP as compared to CHOP alone: pyrexia (56% vs. 46%), lung disorder (31% vs. 24%), cardiac disorder (29% vs. 21%), and chills (13% vs. 4%). In one of these studies (Study 7), more detailed assessment of cardiac toxicity revealed that supraventricular arrhythmias or tachycardia accounted for most of the difference in cardiac disorders, with 4.5% vs. 1.0% incidences for R‑CHOP and CHOP, respectively.
The following Grade 3 or 4 adverse events were reported more frequently among patients in the R‑CHOP arm compared with those in the CHOP arm: thrombocytopenia (9% vs. 7%) and lung disorder (6% vs. 3%). Other severe adverse events reported more commonly among patients receiving R‑CHOP in one or more studies were viral infection, neutropenia and anemia.
Adverse Reactions in Patients with Rheumatoid Arthritis
In general, the adverse events observed in patients with RA were similar in type to those seen in patients with non-Hodgkin’s lymphoma (see WARNINGS, PRECAUTIONS and other sections under ADVERSE REACTIONS). Specific safety considerations in this indication are discussed below.
Where specific percentages are noted, these data are based on 938 patients treated in Phase 2 and 3 studies of Rituxan (2 x 1000 mg) or placebo administered in combination with methotrexate.
| Preferred Term | Placebo + MTX N=398 n (%) | Rituxan + MTX N=540 n (%) |
|---|---|---|
|
||
| Abdominal Pain Upper | 4 (1) | 11 (2) |
| Anxiety | 5 (1) | 9 (2) |
| Arthralgia | 14 (4) | 31 (6) |
| Asthenia | 1 (<1) | 9 (2) |
| Chills | 9 (2) | 16 (3) |
| Dyspepsia | 3 (<1) | 16 (3) |
| Hypercholesterolemia | 1 (<1) | 9 (2) |
| Hypertension | 21 (5) | 43 (8) |
| Migraine | 2 (<1) | 9 (2) |
| Nausea | 19 (5) | 41 (8) |
| Paresthesia | 3 (<1) | 12 (2) |
| Pruritis | 5 (1) | 26 (5) |
| Pyrexia | 8 (2) | 27 (5) |
| Rhinitis | 6 (2) | 14 (3) |
| Throat Irritation | 0 (0) | 11 (2) |
| Upper Respiratory Tract Infection | 23 (6) | 37 (7) |
| Urticaria | 3 (<1) | 12 (2) |
Infusion Reactions
In Rituxan RA placebo‑controlled studies, 32% of Rituxan‑treated patients experienced an adverse event during or within 24 hours following their first infusion, compared to 23% of placebo‑treated patients receiving their first infusion. The incidence of adverse events during the 24‑hour period following the second infusion, Rituxan or placebo, decreased to 11% and 13%, respectively. Acute infusion reactions (manifested by fever, chills, rigors, pruritus, urticaria/rash, angioedema, sneezing, throat irritation, cough, and/or bronchospasm, with or without associated hypotension or hypertension) were experienced by 27% of Rituxan‑treated patients following their first infusion, compared to 19% of placebo‑treated patients receiving their first placebo infusion. The incidence of these acute infusion reactions following the second infusion of Rituxan or placebo decreased to 9% and 11%, respectively. Serious acute infusion reactions were experienced by <1% of patients in either treatment group. Acute infusion reactions required dose modification (stopping, slowing or interruption of the infusion) in 10% and 2% of patients receiving Rituximab or placebo, respectively, after the first course. The proportion of patients experiencing acute infusion reactions decreased with subsequent courses of Rituxan. The administration of IV glucocorticoids prior to Rituxan infusions reduced the incidence and severity of such reactions, however, there was no clear benefit from the administration of oral glucocorticoids for the prevention of acute infusion reactions. Patients in clinical studies also received antihistamines and acetaminophen prior to Rituxan infusions.
Infections
In RA clinical studies, 39% of patients in the Rituxan group experienced an infection of any type compared to 34% of patients in the placebo group. The most common infections were nasopharyngitis, upper respiratory tract infections, urinary tract infections, bronchitis, and sinusitis. The only infections to show an absolute increase over placebo of at least 1% were upper respiratory tract infections, which affected 7% of Rituxan‑treated patients and 6% of placebo‑treated patients and rhinitis, which affected 3% of Rituxan‑treated patients and 2% of placebo‑treated patients.
The incidence of serious infections was 2% in the Rituxan‑treated patients and 1% in the placebo group. One fatal infection (bronchopneumonia) occurred with Rituximab monotherapy during the 24‑weeks placebo‑controlled period in one of the Phase 2 RA studies.
Cardiac Events
The incidence of serious cardiovascular events in the double‑blind part of the clinical trials was 1.7% and 1.3% in Rituxan and placebo treatment groups, respectively. Three cardiovascular deaths occurred during the double‑blind period of the RA studies including all Rituximab regimens (3/769 = 0.4%) as compared to none in the placebo treatment group (0/389).
Since patients with RA are at increased risk for cardiovascular events compared with the general population, patients with RA should be monitored throughout the infusion and Rituxan should be discontinued in the event of a serious or life‑threatening cardiac event.
Immunogenicity
A total of 54/990 patients (5%) with RA tested positive for HACA. Of these, most became positive by week 24. Following the first course, however, some became positive at week 16 or after 24 weeks. Some patients tested positive after the second course of treatment. Limited data are available on the safety or efficacy of Rituxan retreatment in patients who develop HACA. One of 10 HACA‑positive patients who received retreatment with Rituxan experienced a serious acute infusion reaction (bronchospasm). The clinical relevance of HACA formation in Rituximab‑treated patients is unclear.
Post-Marketing Reports
The following adverse reactions have been identified during post-approval use of Rituxan in hematologic malignancies. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to Rituxan.
Hematologic: prolonged pancytopenia, marrow hypoplasia, and late onset neutropenia, hyperviscosity syndrome in Waldenstrom’s macroglobulinemia.
Cardiac: fatal cardiac failure.
Immune/Autoimmune Events: uveitis, optic neuritis, systemic vasculitis, pleuritis, lupus-like syndrome, serum sickness, polyarticular arthritis and vasculitis with rash.
Infection: viral infections, including progressive multifocal leukoencephalopathy (PML), increase in fatal infections in HIV‑associated lymphoma.
Skin: severe mucocutaneous reactions.
Gastrointestinal: bowel obstruction and perforation.
OVERDOSAGE
There has been no experience with overdosage in human clinical trials. Single doses of up to 500 mg/m2 have been given in dose‑escalation clinical trials. 10
DOSAGE AND ADMINISTRATION
Relapsed or Refractory, Low-Grade or Follicular, CD20‑Positive, B‑Cell Non-Hodgkin’s Lymphoma
The recommended dose of Rituxan is 375 mg/m2 IV infusion once weekly for 4 or 8 doses.
Retreatment Therapy
The recommended dose of Rituxan is 375 mg/m2 IV infusion once weekly for 4 doses in responding patients who develop progressive disease after previous Rituxan therapy. Currently there are limited data concerning more than 2 courses.
Previously Untreated, Follicular, CD20‑Positive, B‑cell NHL
The recommended dose of Rituxan is 375 mg/m2 IV infusion, given on Day 1 of each cycle of CVP chemotherapy, for up to 8 doses.
Previously Untreated, Low-Grade, CD20‑Positive, B‑cell NHL
The recommended dose of Rituxan in patients who have not progressed following 6–8 cycles of CVP chemotherapy is 375 mg/m2 IV infusion, once weekly for 4 doses every 6 months for up to 16 doses.
Diffuse Large B‑Cell NHL
The recommended dose of Rituxan is 375 mg/m2 IV per infusion given on Day 1 of each cycle of chemotherapy for up to 8 infusions.
Rheumatoid Arthritis
Rituxan is given as two-1000 mg IV infusions separated by 2 weeks. Glucocorticoids administered as methylprednisolone 100 mg IV or its equivalent 30 minutes prior to each infusion are recommended to reduce the incidence and severity of infusion reactions. Safety and efficacy of retreatment have not been established in controlled trials (see PRECAUTIONS: Retreatment in patients with RA).
Rituxan is given in combination with methotrexate.
Rituxan as a Component of Zevalin® (Ibritumomab tiuxetan) Therapeutic Regimen
As a required component of the Zevalin therapeutic regimen, Rituxan 250 mg/m2 should be infused within 4 hours prior to the administration of Indium‑111‑ (In‑111‑) Zevalin and within 4 hours prior to the administration of Yttrium‑90‑ (Y‑90‑) Zevalin. Administration of Rituxan and In‑111‑Zevalin should precede Rituxan and Y‑90-Zevalin by 7–9 days. Refer to the Zevalin package insert for full prescribing information regarding the Zevalin therapeutic regimen.
Rituxan may be administered in an outpatient setting. DO NOT ADMINISTER AS AN INTRAVENOUS PUSH OR BOLUS. (See Administration).
Instructions for Administration
Preparation for Administration
Use appropriate aseptic technique. Withdraw the necessary amount of Rituxan and dilute to a final concentration of 1 to 4 mg/mL into an infusion bag containing either 0.9% Sodium Chloride, USP, or 5% Dextrose in Water, USP. Gently invert the bag to mix the solution. Discard any unused portion left in the vial. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Rituxan solutions for infusion may be stored at 2°C–8°C (36°F–46°F) for 24 hours. Rituxan solutions for infusion have been shown to be stable for an additional 24 hours at room temperature. However, since Rituxan solutions do not contain a preservative, diluted solutions should be stored refrigerated (2°C–8°C). No incompatibilities between Rituxan and polyvinylchloride or polyethylene bags have been observed.
Administration
DO NOT ADMINISTER AS AN INTRAVENOUS PUSH OR BOLUS
Infusion reactions may occur (see BOXED WARNINGS, WARNINGS, and ADVERSE REACTIONS). Premedication consisting of acetaminophen and an antihistamine should be considered before each infusion of Rituxan. Premedication may attenuate infusion reactions. Since transient hypotension may occur during Rituxan infusion, consideration should be given to withholding antihypertensive medications 12 hours prior to Rituxan infusion.
First Infusion
The Rituxan solution for infusion should be administered intravenously at an initial rate of 50 mg/hr. Rituxan should not be mixed or diluted with other drugs. If infusion reactions do not occur, escalate the infusion rate in 50 mg/hr increments every 30 minutes, to a maximum of 400 mg/hr. If an infusion reaction develops, the infusion should be temporarily slowed or interrupted (see BOXED WARNINGS and WARNINGS). The infusion can continue at one-half the previous rate upon improvement of patient symptoms.
Subsequent Infusions
If the patient tolerated the first infusion well, subsequent Rituxan infusions can be administered at an initial rate of 100 mg/hr, and increased by 100 mg/hr increments at 30‑minute intervals, to a maximum of 400 mg/hr as tolerated. If the patient did not tolerate the first infusion well, follow the guidelines under First Infusion.
Stability and Storage
Rituxan vials are stable at 2°C–8°C (36°F–46°F). Do not use beyond expiration date stamped on carton. Rituxan vials should be protected from direct sunlight. Do not freeze or shake. Refer to the "Preparation for Administration" section for information on the stability and storage of solutions of Rituxan diluted for infusion.
HOW SUPPLIED
Rituxan® (Rituximab) is supplied as 100 mg and 500 mg of sterile, preservative-free, single-use vials.
Single unit 100 mg carton: Contains one 10 mL vial of Rituxan (10 mg/mL).
NDC 50242‑051‑21
Single unit 500 mg carton: Contains one 50 mL vial of Rituxan (10 mg/mL).
NDC 50242‑053‑06
REFERENCES
- Valentine MA, Meier KE, Rossie S, et al. Phosphorylation of the CD20 phosphoprotein in resting B lymphocytes.J Biol Chem 1989;264(19): 11282–7.
- Einfeld DA, Brown JP, Valentine MA, et al. Molecular cloning of the human B cell CD20 receptor predicts a hydrophobic protein with multiple transmembrane domains.EMBO J 1988;7(3):711–7.
- Anderson KC, Bates MP, Slaughenhoupt BL, et al. Expression of human B cell‑associated antigens on leukemias and lymphomas: A model of human B‑cell differentiation. Blood 1984;63(6):1424–33.
- Tedder TF, Boyd AW, Freedman AS, et al. The B cell surface molecule B1 is functionally linked with B‑cell activation and differentiation. J Immunol 1985;135(2):973–9.
- Tedder TF, Zhou LJ, Bell PD, et al. The CD20 surface molecule of B lymphocytes functions as a calcium channel.J Cell Biochem 1990;14D:195.
- Press OW, Applebaum F, Ledbetter JA, Martin PJ, Zarling J, Kidd P, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B-cell lymphomas. Blood 1987;69(2):584–91.
- Dorner, T, Rumester, G. The role of B-cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Op Rheum 2003;15:246-52.
- Reff ME, Carner C, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83(2):435–45.
- Demidem A, Lam T, Alas S, Hariharan K, Hanna N, and Bonavida B. Chimericanti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biotherapy & Radiopharmaceuticals 1997;12(3):177–86.
- Maloney DG, Liles TM, Czerwinski C, Waldichuk J, Rosenberg J, Grillo López A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 1994;84(8):2457–66.
- Berinstein NL, Grillo-López AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum Rituximab (IDEC‑C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Annals of Oncology 1998;9:995–1001.
- Maloney DG, Grillo-López AJ, Bodkin D, White CA, Liles T-M, Royston I, et al. IDEC-C2B8: Results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol 1997;15(10):3266–74.
- Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997;90(6):2188–95.
- McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998;16(8):2825–33.
- Piro LD, White CA, Grillo-López AJ, Janakiraman N, Saven A, Beck TM, et al. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma.Annals of Oncology 1999;10:655–61.
- Data on file.
- Davis TA, White CA, Grillo-López AJ, Velasquez WS, Lin B, Maloney DG, et al. Single-agent monoclonal antibody efficacy in bulky Non-Hodgkin’s lymphoma: results of a phase II trial of rituximab. JCO 1999;17:1851–7.
- Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. Rituximab anti-CD20 monoclonal antibody therapy in non-hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol 2000;18(17):3135–43.
- Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, Izumi H, Ratrie H, Mutasim D, Ariss-Abdo L, Labib RS. Paraneoplastic Pemphigus, an autoimmune mucocutaneous disease associated with neoplasia. NEJM 1990;323(25): 1729–35.
- National Institutes of Health (US), National Cancer Institute. Common Toxicity Criteria. [Bethesda, MD.] : National Institutes of Health, National Cancer Institute; c1998;73p.
Jointly Marketed by: Biogen Idec Inc., and Genentech, Inc.
Rituxan®
(Rituximab)
Manufactured by: 4835500
Genentech, Inc.
1 DNA Way
South San Francisco, CA 94080-4990
Initial US Approval November 26, 1997
Revision Date February 21, 2007
©2007 Biogen Idec, Inc. and Genentech,
Inc.
Patient Information
Rituxan®
(ri-tuk'-san)
(Rituximab)
Read this patient information leaflet when you have been prescribed Rituxan and each time you are scheduled to receive a Rituxan infusion. This information does not take the place of talking to your doctor about your medical condition or your treatment. Talk with your doctor if you have any questions about your treatment with Rituxan.
What is the most important safety information I should know about Rituxan?
Rituxan can cause the following serious side effects, some of which could be life-threatening:
- Infusion reactions. Tell your doctor or get medical treatment right away if you get hives, swelling, dizziness, blurred vision, drowsiness, headache, cough, wheezing, or have trouble breathing while receiving or after receiving Rituxan.
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of certain blood cancers. TLS can cause kidney failure and the need for dialysis treatment. Patients receiving Rituxan for non‑Hodgkin’s lymphoma may get TLS.
- Severe skin reactions. Tell your doctor or get medical treatment right away if you get painful sores, ulcers, blisters, or peeling skin while receiving or after receiving Rituxan.
- Progressive Multifocal Leukoencephalopathy (PML). PML is a rare brain infection that usually causes death or severe disability.
- PML has been reported in patients during or after their treatment with Rituxan.
- There is no known treatment, prevention, or cure for PML.
- Call your doctor right away if you notice any new or worsening medical problems, such as a new or sudden change in thinking, walking, strength, vision, or other problems that have lasted over several days.
Also, see “What are possible side-effects with Rituxan?” for other serious side effects, some of which could be life-threatening.
What is Rituxan?
Rituxan is a biologic medicine used in adults:
- alone or with other anti‑cancer medicines to treat certain types of non‑Hodgkin’s lymphoma (NHL).
- with another medicine called methotrexate to reduce the signs and symptoms of Rheumatoid Arthritis (RA) after at least one other medicine called a tumor necrosis factor (TNF) inhibitor has been used and did not work well.
Rituxan has not been studied in children.
How does Rituxan work?
Rituxan works by getting rid of certain B‑cells in the blood. B‑cells are a type of white blood cell found in the blood. B‑cells usually help the body fight infection. B‑cells play an important role in diseases such as NHL and RA. Rituxan may also get rid of healthy B‑cells and this can give you a higher chance for getting infections.
Who should not receive
Rituxan?
Do not receive Rituxan if you ever had an allergic reaction to Rituxan
What should I tell my doctor
before treatment with Rituxan?
Tell your doctor about all of your medical conditions, including if you:
- have an infection or have an infection that will not go away or that keeps coming back.
- are scheduled to have surgery.
- have had hepatitis B virus infection or are a carrier of hepatitis B virus. Your doctor should check you closely for signs of a hepatitis infection during treatment with Rituxan and for several months after treatment ends.
- have any scheduled vaccinations. It is not known if Rituxan affects your ability to respond to vaccines.
- have heart or lung problems.
- are pregnant or planning to become pregnant. It is not known if Rituxan can harm your unborn baby.
- are breastfeeding. It is not known if Rituxan passes into human breast milk. You should not breastfeed while being treated with Rituxan.
Tell your doctor about all the other medicines you take, including prescription and nonprescription medicines, vitamins, or herbal supplements. If you have RA, tell your doctor if you are taking or took another biologic medicine called a TNF inhibitor or a DMARD (disease modifying anti‑rheumatic drug).
How do I receive Rituxan?
- Rituxan is given through a needle placed in a vein (IV infusion), in your arm. Rituxan therapy is given in different ways for NHL and RA. Talk to your doctor about how you will receive Rituxan.
- Your doctor may prescribe other medicines before each infusion of Rituxan to prevent or reduce pain, or to reduce fever and allergic reactions.
- Your doctor should do regular blood tests to check for side effects or reactions to Rituxan.
What are possible side effects with Rituxan?
Rituxan can cause the following serious side effects, some of which could be life-threatening side effects, including (See “What is the most important safety information I should know about Rituxan?”)
- Infusion reactions
- Tumor Lysis Syndrome (TLS)
- Severe skin reactions
- Progressive Multifocal Leukoencephalopathy (PML)
Other serious side effects with Rituxan include:
- Hepatitis B virus reactivation. Tell your doctor if you had Hepatitis B virus or are a carrier of Hepatitis B virus. Rituxan may make you sick with Hepatitis B virus again and cause serious liver problems. People with active liver disease due to Hepatitis B should stop receiving Rituxan.
- Heart Problems. Tell your doctor about any heart problems you have including chest pain (angina) and irregular heart beats. Rituxan can cause chest pain and irregular heart beats which may require treatment.
- Infections. Rituxan can increase your chances for getting infections. Call your doctor right away if you have a persistent cough, fever, chills, congestion, or any flu-like symptoms while receiving Rituxan. These symptoms may be signs of a serious infection.
- Stomach and bowel problems. Serious stomach and bowel problems have been seen when Rituxan has been used with anti-cancer medicines in some patients with non-Hodgkin’s lymphoma. Call your doctor right away if you have any stomach area pain during treatment with Rituxan.
Common side effects with Rituxan include:
Fever, chills, shakes, itching, hives, sneezing, swelling, throat irritation or tightness, and cough. These usually occur within 24 hours after the first infusion. Other common side effects include headache, nausea, upper respiratory tract infection, and aching joints. If you have any of these symptoms, tell your doctor or nurse.
What if I still have questions?
If you have any questions about Rituxan or your health, talk with your doctor. You can also visit the Rituxan internet sites at www.Rituxan.com or the companies’ internet sites at www.Gene.com or www.Biogenidec.com or call 1‑877‑4‑Rituxan (877‑474‑8892).
Jointly Marketed by: Biogen Idec Inc. and Genentech, Inc.
Manufactured by:
Genentech, Inc.
1 DNA Way
South San Francisco, CA
94080-4990
©2007 Biogen Idec Inc. and Genentech, Inc.
Patient Information Approval February 21, 2007
| Rituxan (rituximab) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Rituxan (rituximab) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 03/2007