prevpac (lansoprazole, amoxicillin and clarithromycin)
[TAP Pharmaceutical Products Inc.]
To reduce the development of drug-resistant bacteria and maintain the effectiveness of PREVPAC® and other antibacterial drugs, PREVPAC should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
THESE PRODUCTS ARE INTENDED ONLY FOR USE AS DESCRIBED. The individual products contained in this package should not be used alone or in combination for other purposes. The information described in this labeling concerns only the use of these products as indicated in this daily administration pack. For information on use of the individual components when dispensed as individual medications outside this combined use for treating Helicobacter pylori (H. pylori), please see the package inserts for each individual product.
DESCRIPTION
PREVPAC consists of a daily administration pack containing two PREVACID 30-mg capsules, four amoxicillin 500-mg capsules, USP, and two clarithromycin 500-mg tablets, USP, for oral administration.
PREVACID® (lansoprazole) Delayed-Release Capsules
The active ingredient in PREVACID capsules is a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F3N3O2S with a molecular weight of 369.37. The structural formula is:
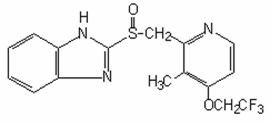
Lansoprazole is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water.
Each delayed-release capsule contains enteric-coated granules consisting of lansoprazole (30 mg), hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, colloidal silicon dioxide, magnesium carbonate, methacrylic acid copolymer, starch, talc, sugar sphere, sucrose, polyethylene glycol, polysorbate 80, and titanium dioxide. Components of the gelatin capsule include gelatin, titanium dioxide, D&C Red No. 28, FD&C Blue No. 1, and FD&C Red No. 40.
Amoxicillin Capsules, USP
Amoxicillin is a semisynthetic antibiotic, an analogue of ampicillin, with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microorganisms. Chemically it is (2S, 5R, 6R)-6-[(R)-(-)-2-amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0] heptane-2-carboxylic acid trihydrate. Its empirical formula is C16H19N3O5S • 3H2O with a molecular weight of 419.45. It has the following chemical structure:
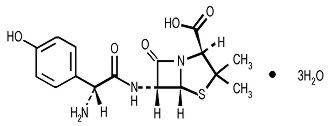
Amoxicillin capsules are intended for oral administration. The yellow opaque capsules contain amoxicillin trihydrate equivalent to 500 mg of amoxicillin.
Inactive ingredients: Capsule shells - yellow ferric oxide, titanium dioxide, gelatin, black ferric oxide; Capsule contents – cellulose microcrystalline and magnesium stearate.
BIAXIN® Filmtab® (clarithromycin tablets, USP)
Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-0-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is:
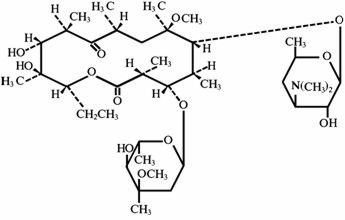
Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water. Each yellow oval film-coated immediate-release tablet contains 500 mg of clarithromycin and the following inactive ingredients: hypromellose, hydroxypropyl cellulose, colloidal silicon dioxide, croscarmellose sodium, D&C Yellow No. 10, magnesium stearate, microcrystalline cellulose, povidone, propylene glycol, sorbic acid, sorbitan monooleate, titanium dioxide, and vanillin.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Pharmacokinetics when all three of the PREVPAC components (PREVACID capsules, amoxicillin capsules, clarithromycin tablets) were coadministered has not been studied. Studies have shown no clinically significant interactions of PREVACID and amoxicillin or PREVACID and clarithromycin when administered together. There is no information about the gastric mucosal concentrations of PREVACID, amoxicillin and clarithromycin after administration of these agents concomitantly. The systemic pharmacokinetic information presented below is based on studies in which each product was administered alone.
PREVACID
PREVACID capsules contain an enteric-coated granule formulation of lansoprazole. Absorption of lansoprazole begins only after the granules leave the stomach. Absorption is rapid, with mean peak plasma levels of lansoprazole occurring after approximately 1.7 hours. Peak plasma concentrations of lansoprazole (Cmax) and the area under the plasma concentration curve (AUC) of lansoprazole are approximately proportional in doses from 15 mg to 60 mg after single-dose oral administration. Lansoprazole does not accumulate and its pharmacokinetics are unaltered by multiple dosing.
The absorption of lansoprazole is rapid, with mean Cmax occurring approximately 1.7 hours after oral dosing, and relatively complete with absolute bioavailability over 80%. In healthy subjects, the mean (± SD) plasma half-life was 1.5 (± 1.0) hours. Both Cmax and AUC are diminished by about 50% if the drug is given 30 minutes after food as opposed to the fasting condition. There is no significant food effect if the drug is given before meals.
Lansoprazole is 97% bound to plasma proteins. Plasma protein binding is consistent over the concentration range of 0.05 to 5.0 mcg/mL.
Lansoprazole is extensively metabolized in the liver. Two metabolites have been identified in measurable quantities in plasma (the hydroxylated sulfinyl and sulfone derivatives of lansoprazole). These metabolites have very little or no antisecretory activity. Lansoprazole is thought to be transformed into two active species which inhibit acid secretion by (H+,K+)-ATPase within the parietal cell canaliculus, but are not present in the systemic circulation. The plasma elimination half-life of lansoprazole does not reflect its duration of suppression of gastric acid secretion. Thus, the plasma elimination half-life is less than two hours while the acid inhibitory effect lasts more than 24 hours.
Following single-dose oral administration of PREVACID, virtually no unchanged lansoprazole was excreted in the urine. In one study, after a single oral dose of 14C-lansoprazole, approximately one-third of the administered radiation was excreted in the urine and two-thirds was recovered in the feces. This implies a significant biliary excretion of the metabolites of lansoprazole.
Special Populations
Geriatric
The clearance of lansoprazole is decreased in the elderly, with elimination half-life increased approximately 50% to 100%. Because the mean half-life in the elderly remains between 1.9 to 2.9 hours, repeated once daily dosing does not result in accumulation of lansoprazole. Peak plasma levels were not increased in the elderly.
Renal Insufficiency
In patients with severe renal insufficiency, plasma protein binding decreased by 1.0%-1.5% after administration of 60 mg of lansoprazole. Patients with renal insufficiency had a shortened elimination half-life and decreased total AUC (free and bound). AUC for free lansoprazole in plasma, however, was not related to the degree of renal impairment, and Cmax and Tmax were not different from subjects with healthy kidneys.
Hepatic Insufficiency
In patients with various degrees of chronic hepatic disease, the mean plasma half-life of the drug was prolonged from 1.5 hours to 3.2-7.2 hours. An increase in mean AUC of up to 500% was observed at steady state in hepatically-impaired patients compared to healthy subjects. Dose reduction in patients with severe hepatic disease should be considered.
Race
The pooled pharmacokinetic parameters of PREVACID from twelve U.S. Phase I studies (N=513) were compared to the mean pharmacokinetic parameters from two Asian studies (N=20). The mean AUCs of PREVACID in Asian subjects are approximately twice that seen in pooled U.S. data; however, the inter-individual variability is high. The Cmax values are comparable.
Amoxicillin
Amoxicillin is stable in the presence of gastric acid and may be given without regard to meals. It is rapidly absorbed after oral administration. It diffuses readily into most body tissues and fluids, with the exception of brain and spinal fluid, except when meninges are inflamed. The half-life of amoxicillin is 61.3 minutes. Most of the amoxicillin is excreted unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid. In blood serum, amoxicillin is approximately 20% protein-bound.
Orally administered doses of 500 mg amoxicillin capsules result in average peak blood levels 1 to 2 hours after administration in the range of 5.5 mcg/mL to 7.5 mcg/mL.
Detectable serum levels are observed up to 8 hours after an orally administered dose of amoxicillin. Approximately 60% of an orally administered dose of amoxicillin is excreted in the urine within 6 to 8 hours.
Clarithromycin
Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250 mg clarithromycin tablets was approximately 50%. For a single 500 mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability. Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly increase the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentration-time curve (AUC). Therefore, BIAXIN tablets may be given without regard to food.
In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 3 to 4 µg/mL with a 500-mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was 5 to 7 hours with 500 mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended dose of 500 mg administered every 8 to 12 hours. With a 500-mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is up to 1 µg/mL, and its elimination half-life is about 7 to 9 hours. The steady-state concentration of this metabolite is generally attained within 3 to 4 days.
After a 500-mg tablet every 12 hours, the urinary excretion of clarithromycin is approximately 30%. The renal clearance of clarithromycin approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with a 500-mg tablet administered every 12 hours.
The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Pharmacodynamics
MICROBIOLOGY
Lansoprazole, clarithromycin and/or amoxicillin have been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Helicobacter
Helicobacter pylori
Pretreatment Resistance
Clarithromycin pretreatment resistance (≥2.0 µg/mL) was 9.5% (91/960) by E-test and 11.3% (12/106) by agar dilution in the dual and triple therapy clinical trials (M93-125, M93-130, M93-131, M95-392, and M95-399).
Amoxicillin pretreatment susceptible isolates (≤0.25 µg/mL) occurred in 97.8% (936/957) and 98.0% (98/100) of the patients in the dual and triple therapy clinical trials by E-test and agar dilution, respectively. Twenty-one of 957 patients (2.2%) by E-test and 2 of 100 patients (2.0%) by agar dilution had amoxicillin pretreatment MICs of >0.25 µg/mL. One patient on the 14-day triple therapy regimen had an unconfirmed pretreatment amoxicillin minimum inhibitory concentration (MIC) of >256 µg/mL by E-test and the patient was eradicated of H. pylori.
| Clarithromycin Pretreatment Results | Clarithromycin Post-treatment Results | |||||
|---|---|---|---|---|---|---|
| H. pylori negative –eradicated | H. pylori positive –not eradicated Post-treatment susceptibility results |
|||||
| S† | I† | R† | No MIC | |||
| Triple Therapy 14-Day (lansoprazole 30 mg b.i.d./amoxicillin 1 gm b.i.d./clarithromycin 500 mg b.i.d.) (M95-399, M93-131, M95-392) | ||||||
| Susceptible† | 112 | 105 | 7 | |||
| Intermediate† | 3 | 3 | ||||
| Resistant† | 17 | 6 | 7 | 4 | ||
| Triple Therapy 10-Day (lansoprazole 30 mg b.i.d./amoxicillin 1 gm b.i.d./clarithromycin 500 mg b.i.d.) (M95-399) | ||||||
| Susceptible† | 42 | 40 | 1 | 1 | ||
| Intermediate† | ||||||
| Resistant† | 4 | 1 | 3 | |||
Patients not eradicated of H. pylori following lansoprazole/amoxicillin/clarithromycin triple therapy will likely have clarithromycin resistant H. pylori. Therefore, for those patients who fail therapy, clarithromycin susceptibility testing should be done when possible. Patients with clarithromycin resistant H. pylori should not be treated with lansoprazole/amoxicillin/clarithromycin triple therapy or with regimens which include clarithromycin as the sole antimicrobial agent.
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
In the dual and triple therapy clinical trials, 82.6% (195/236) of the patients that had pretreatment amoxicillin susceptible MICs (≤0.25 µg/mL) were eradicated of H. pylori. Of those with pretreatment amoxicillin MICs of >0.25 µg/mL, three of six had the H. pylori eradicated. A total of 30% (21/70) of the patients failed lansoprazole 30 mg t.i.d./amoxicillin 1 gm t.i.d. dual therapy and a total of 12.8% (22/172) of the patients failed the 10- and 14-day triple therapy regimens. Post-treatment susceptibility results were not obtained on 11 of the patients who failed therapy. Nine of the 11 patients with amoxicillin post-treatment MICs that failed the triple therapy regimen also had clarithromycin resistant H. pylori isolates.
Susceptibility Test for Helicobacter pylori
The reference methodology for susceptibility testing of H. pylori is agar dilution MICs.1 One to three microliters of an inoculum equivalent to a No. 2 McFarland standard (1 × 107– 1 × 108 CFU/mL for H. pylori) are inoculated directly onto freshly prepared antimicrobial containing Mueller-Hinton agar plates with 5% aged defibrinated sheep blood (≥ 2 weeks old). The agar dilution plates are incubated at 35°C in a microaerobic environment produced by a gas generating system suitable for Campylobacter species. After 3 days of incubation, the MICs are recorded as the lowest concentration of antimicrobial agent required to inhibit growth of the organism. The clarithromycin and amoxicillin MIC values should be interpreted according to the following criteria:
| Clarithromycin MIC (µg/mL)* | Interpretation |
| ≤0.25 | Susceptible (S) |
| 0.5-1.0 | Intermediate (I) |
| ≥2.0 | Resistant (R) |
| Amoxicillin MIC (µg/mL)† | Interpretation |
| ≤0.25 | Susceptible (S) |
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard clarithromycin and amoxicillin powders should provide the following MIC values:
| Microorganisms | Antimicrobial Agent | MIC (µg/mL)* |
|---|---|---|
|
||
| H. pylori ATCC 43504 | Clarithromycin | 0.015-0.12 µg/mL |
| H. pylori ATCC 43504 | Amoxicillin | 0.015-0.12 µg/mL |
Antisecretory activity
After oral administration, lansoprazole was shown to significantly decrease the basal acid output and significantly increase the mean gastric pH and percent of time the gastric pH was >3 and >4. Lansoprazole also significantly reduced meal-stimulated gastric acid output and secretion volume, as well as pentagastrin-stimulated acid output. In patients with hypersecretion of acid, lansoprazole significantly reduced basal and pentagastrin-stimulated gastric acid secretion. Lansoprazole inhibited the normal increases in secretion volume, acidity and acid output induced by insulin.
In a crossover study that included lansoprazole 15 and 30 mg for five days, the following effects on intragastric pH were noted:
| PREVACID | |||||
|---|---|---|---|---|---|
| Baseline | 15 mg | 30 mg | |||
| Parameter | Value | Day 1 | Day 5 | Day 1 | Day 5 |
| NOTE: An intragastric pH of >4 reflects a reduction in gastric acid by 99%. | |||||
| Mean 24-Hour pH | 2.1 | 2.7* | 4.0* | 3.6† | 4.9† |
| Mean Nighttime pH | 1.9 | 2.4 | 3.0* | 2.6 | 3.8† |
| % Time Gastric pH>3 | 18 | 33* | 59* | 51† | 72† |
| % Time Gastric pH>4 | 12 | 22* | 49* | 41† | 66† |
After the initial dose in this study, increased gastric pH was seen within 1-2 hours with lansoprazole 30 mg and 2-3 hours with lansoprazole 15 mg. After multiple daily dosing, increased gastric pH was seen within the first hour postdosing with lansoprazole 30 mg and within 1-2 hours postdosing with lansoprazole 15 mg.
Acid suppression may enhance the effect of antimicrobials in eradicating Helicobacter pylori (H. pylori). The percentage of time gastric pH was elevated above 5 and 6 was evaluated in a crossover study of PREVACID given q.d., b.i.d. and t.i.d.
| PREVACID | ||||
|---|---|---|---|---|
| Parameter | 30 mg q.d. | 15 mg b.i.d. | 30 mg b.i.d. | 30 mg t.i.d. |
| % Time Gastric pH>5 | 43 | 47 | 59* | 77† |
| % Time Gastric pH>6 | 20 | 23 | 28 | 45† |
The inhibition of gastric acid secretion as measured by intragastric pH returns gradually to normal over two to four days after multiple doses. There is no indication of rebound gastric acidity.
CLINICAL STUDIES
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Randomized, double-blind clinical studies performed in the U.S. in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) evaluated the efficacy of PREVPAC as triple 14-day therapy for the eradication of H. pylori. The triple therapy regimen (PREVACID 30 mg b.i.d. plus amoxicillin 1 gm b.i.d. plus clarithromycin 500 mg b.i.d.) produced statistically significantly higher eradication rates than PREVACID plus amoxicillin, PREVACID plus clarithromycin, and amoxicillin plus clarithromycin dual therapies.
H. pylori eradication was defined as two negative tests (culture and histology) at 4 - 6 weeks following the end of treatment.
Triple therapy was shown to be more effective than all possible dual therapy combinations. The combination of PREVACID plus amoxicillin and clarithromycin as triple therapy was effective in eradicating H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
A randomized, double-blind clinical study performed in the U.S. in patients with H. pylori and duodenal ulcer disease (defined as an active ulcer or history of an ulcer within one year) compared the efficacy of PREVACID triple therapy for 10 and 14 days. This study established that the 10-day triple therapy was equivalent to the 14-day triple therapy in eradicating H. pylori.
| (PREVACID/amoxicillin/clarithromycin) Percent of Patients Cured [95% Confidence Interval] (Number of patients) |
|||
|---|---|---|---|
| Study | Duration | Triple Therapy Evaluable Analysis* | Triple Therapy Intent-to-Treat Analysis† |
|
|||
| M93-131 | 14 days | 92‡
[80.0-97.7] (N=48) | 86‡
[73.3-93.5] (N=55) |
| M95-392 | 14 days | 86§
[75.7-93.6] (N=66) | 83§
[72.0-90.8] (N=70) |
| M95-399¶ | 14 days | 85 [77.0-91.0] (N=113) | 82 [73.9-88.1] (N=126) |
| 10 days | 84 [76.0-89.8] (N=123) | 81 [73.9-87.6] (N=135) |
|
INDICATIONS AND USAGE
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
The components in PREVPAC (PREVACID, amoxicillin, and clarithromycin) are indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence (See CLINICAL STUDIES and DOSAGE AND ADMINISTRATION).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of PREVPAC and other antibacterial drugs, PREVPAC should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
PREVPAC is contraindicated in patients with known hypersensitivity to any component of the formulation of PREVACID, any macrolide antibiotic, or any penicillin.
Concomitant administration of PREVPAC with cisapride, pimozide, astemizole, terfenadine, ergotamine or dihydroergotamine is contraindicated. There have been post-marketing reports of drug interactions when clarithromycin and/or erythromycin are co-administered with cisapride, pimozide, astemizole, or terfenadine resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) most likely due to inhibition of metabolism of these drugs by erythromycin and clarithromycin. Fatalities have been reported.
(Please refer to full prescribing information for amoxicillin and clarithromycin before prescribing).
WARNINGS
Amoxicillin
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH AMOXICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, AMOXICILLIN SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED.
SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Clarithromycin
CLARITHROMYCIN SHOULD NOT BE USED IN PREGNANT WOMEN EXCEPT IN CLINICAL CIRCUMSTANCES WHERE NO ALTERNATIVE THERAPY IS APPROPRIATE. IF PREGNANCY OCCURS WHILE TAKING CLARITHROMYCIN, THE PATIENT SHOULD BE APPRISED OF THE POTENTIAL HAZARD TO THE FETUS. CLARITHROMYCIN HAS DEMONSTRATED ADVERSE EFFECTS OF PREGNANCY OUTCOME AND/OR EMBRYO-FETAL DEVELOPMENT IN MONKEYS, RATS, MICE, AND RABBITS AT DOSES THAT PRODUCED PLASMA LEVELS 2 TO 17 TIMES THE SERUM LEVELS ACHIEVED IN HUMANS TREATED AT THE MAXIMUM RECOMMENDED HUMAN DOSES. (See PRECAUTIONS - Pregnancy).
There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients. (See PRECAUTIONS).
Amoxicillin and/or Clarithromycin
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clarithromycin and amoxicillin, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated colitis."
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
PRECAUTIONS
Clarithromycin is principally excreted via the liver and kidney. Clarithromycin may be administered without dosage adjustment to patients with hepatic impairment and normal renal function. However, in the presence of severe renal impairment with or without coexisting hepatic impairment, decreased dosage or prolonged dosing intervals may be appropriate.
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur, PREVPAC should be discontinued and appropriate therapy instituted.
Symptomatic response to therapy with PREVACID does not preclude the presence of gastric malignancy.
Prescribing PREVPAC in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Each dose of PREVPAC contains four pills: one pink and black capsule (PREVACID), two opaque, yellow capsules (amoxicillin) and one yellow tablet (clarithromycin). Each dose should be taken twice per day before eating. Patients should be instructed to swallow each pill whole.
Biaxin may interact with some drugs; therefore patients should be advised to report to their doctor the use of any other medications.
Patients should be counseled that antibacterial drugs including PREVPAC should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When PREVPAC is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by PREVPAC or other antibacterial drugs in the future.
Drug Interactions
PREVACID
PREVACID causes long-lasting inhibition of gastric acid secretion. PREVACID substantially decreases the systemic concentrations of the HIV protease inhibitor atazanavir, which is dependent upon the presence of gastric acid for absorption, and may result in a loss of therapeutic effect of atazanavir and the development of HIV resistance. Therefore, PREVACID, or other proton pump inhibitors, should not be co-administered with atazanavir.
It is theoretically possible that PREVACID may also interfere with the absorption of other drugs where gastric pH is an important determinant of bioavailability (e.g., ketoconazole, ampicillin esters, iron salts, digoxin).
PREVACID is metabolized through the cytochrome P450 system, specifically through the CYP3A and CYP2C19 isozymes. Studies have shown that PREVACID does not have clinically significant interactions with other drugs metabolized by the cytochrome P450 system, such as warfarin, antipyrine, indomethacin, ibuprofen, phenytoin, propranolol, prednisone, diazepam, or clarithromycin in healthy subjects. These compounds are metabolized through various cytochrome P450 isozymes including CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A. When PREVACID was administered concomitantly with theophylline (CYP1A2, CYP3A), a minor increase (10%) in the clearance of theophylline was seen. Because of the small magnitude and the direction of the effect on theophylline clearance, this interaction is unlikely to be of clinical concern. Nonetheless, individual patients may require additional titration of their theophylline dosage when PREVACID is started or stopped to ensure clinically effective blood levels.
In a study of healthy subjects neither the pharmacokinetics of warfarin enantiomers nor prothrombin time were affected following single or multiple 60 mg doses of lansoprazole. However, there have been reports of increased International Normalized Ratio (INR) and prothrombin time in patients receiving proton pump inhibitors, including PREVACID, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time.
PREVACID has also been shown to have no clinically significant interaction with amoxicillin.
In a single-dose crossover study examining PREVACID 30 mg and omeprazole 20 mg each administered alone and concomitantly with sucralfate 1 gram, absorption of the proton pump inhibitors was delayed and their bioavailability was reduced by 17% and 16%, respectively, when administered concomitantly with sucralfate. Therefore, proton pump inhibitors should be taken at least 30 minutes prior to sucralfate. In clinical trials, antacids were administered concomitantly with PREVACID Delayed-Release Capsules; this did not interfere with its effect.
Amoxicillin
Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of amoxicillin and probenecid may result in increased and prolonged blood levels of amoxicillin.
Chloramphenicol, macrolides, sulfonamides, and tetracyclines may interfere with bactericidal effects of penicillin. This has been demonstrated in vitro; however, the clinical significance of this interaction is not well documented.
Clarithromycin
Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range. In two studies in which theophylline was administered with clarithromycin (a theophylline sustained-release formulation was dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg q12h clarithromycin), the steady-state levels of Cmax, Cmin, and the area under the serum concentration time curve (AUC) of theophylline increased about 20%.
Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered.
When clarithromycin and terfenadine were coadministered, plasma concentrations of the active acid metabolite of terfenadine were threefold higher, on average, than the values observed when terfenadine was administered alone. The pharmacokinetics of clarithromycin and the 14-hydroxy-clarithromycin were not significantly affected by coadministration of terfenadine once clarithromycin reached steady-state conditions. Concomitant administration of clarithromycin with terfenadine is contraindicated. (See CONTRAINDICATIONS).
Spontaneous reports in the post-marketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously.
Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have also been reported in post-marketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin levels should be carefully monitored while patients are receiving digoxin and clarithromycin simultaneously.
Colchicine is a substrate for both CYP3A and the efflux transporter, P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered together, inhibition of Pgp and/or CYP3A by clarithromycin may lead to increased exposure to colchicine. Patients should be monitored for clinical symptoms of colchicine toxicity. (See WARNINGS).
Erythromycin and clarithromycin are substrates and inhibitors of the 3A isoform subfamily of the cytochrome P450 enzyme system (CYP3A). Coadministration of erythromycin or clarithromycin and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both the therapeutic and adverse effects of the concomitant drug. Dosage adjustments may be considered, and when possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored closely in patients concurrently receiving clarithromycin or erythromycin.
The following are examples of some clinically significant CYP3A based drug interactions. Interactions with other drugs metabolized by the CYP3A isoform are also possible. Increased serum concentrations of carbamazepine and the active acid metabolite of terfenadine were observed in clinical trials with clarithromycin.
The following CYP3A based drug interactions have been observed with erythromycin products and/or with clarithromycin in post-marketing experience:
Antiarrhythmics
There have been post-marketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during coadministration of clarithromycin with these drugs. Serum levels of these medications should also be monitored.
Ergotamine/dihydroergotamine
Post-marketing reports indicate that coadministration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. Concomitant administration of clarithromycin with ergotamine or dihydroergotamine is contraindicated (See CONTRAINDICATIONS ).
Triazolobenziodidiazepines (such as triazolam and alprazolam) and related benzodiazepines (such as midazolam)
Erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines. There have been postmarketing reports of drug interactions and CNS effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam.
HMG-CoA Reductase Inhibitors
As with other macrolides, clarithromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (e.g., lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
Sildenafil (Viagra)
Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. A similar interaction may occur with clarithromycin; reduction of sildenafil dosage should be considered. (See Viagra package insert.) There have been spontaneous or published reports of CYP3A based interactions of erythromycin and/or clarithromycin with cyclosporine, carbamazepine, tacrolimus, alfentanil, disopyramide, rifabutin, quinidine, methylprednisolone, cilostazol, and bromocriptine.
Concomitant administration of clarithromycin with cisapride, pimozide, astemizole, or terfenadine is contraindicated (see CONTRAINDICATIONS).
In addition, there have been reports of interactions of erythromycin or clarithromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
For information on interactions between clarithromycin in combination with other drugs which may be administered to HIV-infected patients, see the BIAXIN package insert, Drug Interactions, under the PRECAUTIONS section.
Drug/Laboratory Test Interactions
High urine concentrations of ampicillin may result in false-positive reactions when testing for the presence of glucose in urine using Clinitest®, Benedict's Solution or Fehling's Solution. Since this effect may also occur with amoxicillin, it is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as Clinistix®) be used. Following administration of ampicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted. This effect may also occur with amoxicillin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
PREVACID
In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated orally with doses of 5 to 150 mg/kg/day, about 1 to 40 times the exposure on a body surface (mg/m2) basis, of a 50-kg person of average height (1.46 m2 body surface area) given the recommended human dose of 30 mg/day (22.2 mg/m2). Lansoprazole produced dose-related gastric enterochromaffin-like (ECL) cell hyperplasia and ECL cell carcinoids in both male and female rats. It also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg/kg/day (4 to 40 times the recommended human dose based on body surface area) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat. Testicular interstitial cell adenoma also occurred in 1 of 30 rats treated with 50 mg/kg/day (13 times the recommended human dose based on body surface area) in a 1-year toxicity study.
In a 24-month carcinogenicity study, CD-1 mice were treated orally with doses of 15 to 600 mg/kg/day, 2 to 80 times the recommended human dose based on body surface area. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg/kg/day (40 to 80 times the recommended human dose based on body surface area) and female mice treated with 150 to 600 mg/kg/day (20 to 80 times the recommended human dose based on body surface area) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg/kg/day (10 to 80 times the recommended human dose based on body surface area).
Lansoprazole was not genotoxic in the Ames test, the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test or the rat bone marrow cell chromosomal aberration test. It was positive in in vitro human lymphocyte chromosomal aberration assays.
Lansoprazole at oral doses up to 150 mg/kg/day (40 times the recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
Amoxicillin
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of amoxicillin alone have not been conducted.
Clarithromycin
The following in vitro mutagenicity tests have been conducted with clarithromycin:
Salmonella/Mammalian Microsomes Test
Bacterial Induced Mutation Frequency Test
In Vitro Chromosome Aberration Test
Rat Hepatocyte DNA Synthesis Assay
Mouse Lymphoma Assay
Mouse Dominant Lethal Study
Mouse Micronucleus Test
All tests had negative results except the In Vitro Chromosome Aberration Test which was weakly positive in one test and negative in another.
In addition, a Bacterial Reverse-Mutation Test (Ames Test) has been performed on clarithromycin metabolites with negative results.
Fertility and reproduction studies have shown that daily doses of up to 160 mg/kg/day (1.3 times the recommended maximum human dose based on mg/m2) to male and female rats caused no adverse effects on the estrous cycle, fertility, parturition, or number and viability of offspring. Plasma levels in rats after 150 mg/kg/day were 2 times the human serum levels.
In the 150 mg/kg/day monkey studies, plasma levels were 3 times the human serum levels. When given orally at 150 mg/kg/day (2.4 times the recommended maximum human dose based on mg/m2), clarithromycin was shown to produce embryonic loss in monkeys. This effect has been attributed to marked maternal toxicity of the drug at this high dose.
In rabbits, in utero fetal loss occurred at an intravenous dose of 33 mg/m2, which is 17 times less than the maximum proposed human oral daily dose of 618 mg/m2.
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of clarithromycin.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Category C is based on the pregnancy category for clarithromycin.
Four teratogenicity studies in rats (three with oral doses and one with intravenous doses up to 160 mg/kg/day administered during the period of major organogenesis) and two in rabbits at oral doses up to 125 mg/kg/day (approximately 2 times the recommended maximum human dose based on mg/m2) or intravenous doses of 30 mg/kg/day administered during gestation days 6 to 18 failed to demonstrate any teratogenicity from clarithromycin. Two additional oral studies in a different rat strain at similar doses and similar conditions demonstrated a low incidence of cardiovascular anomalies at doses of 150 mg/kg/day administered during gestation days 6 to 15. Plasma levels after 150 mg/kg/day were 2 times the human serum levels. Four studies in mice revealed a variable incidence of cleft palate following oral doses of 1000 mg/kg/day (2 and 4 times the recommended maximum human dose based on mg/m2, respectively) during gestation days 6 to 15. Cleft palate was also seen at 500 mg/kg/day. The 1000 mg/kg/day exposure resulted in plasma levels 17 times the human serum levels. In monkeys, an oral dose of 70 mg/kg/day (an approximate equidose of the recommended maximum human dose based on mg/m2) produced fetal growth retardation at plasma levels that were 2 times the human serum levels.
There were no adequate and well-controlled studies of PREVPAC in pregnant women. PREVPAC should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (See WARNINGS).
Labor and Delivery
Oral ampicillin-class antibiotics are poorly absorbed during labor. Studies in guinea pigs showed that intravenous administration of ampicillin slightly decreased the uterine tone and frequency of contractions, but moderately increased the height and duration of contractions. However, it is not known whether use of these drugs in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Lansoprazole or its metabolites are excreted in the milk of rats. It is not known whether lansoprazole is excreted in human milk. Penicillins have been shown to be excreted in human milk. Amoxicillin use by nursing mothers may lead to sensitization of infants. Caution should be exercised when amoxicillin is administered to a nursing woman. It is not known whether clarithromycin is excreted in human milk. It is known that clarithromycin is excreted in the milk of lactating animals and that other drugs of this class are excreted in human milk.
Due to the potential for serious adverse reactions in nursing infants from PREVPAC, and the potential for tumorigenicity shown for lansoprazole in rat carcinogenicity studies, a decision should be made whether to discontinue nursing or to discontinue PREVPAC, taking into account the importance of the therapy to the mother.
Pediatric Use
Safety and effectiveness of PREVPAC in pediatric patients infected with H. pylori have not been established. (See CONTRAINDICATIONS and WARNINGS).
Use in Geriatric Patients
Elderly patients may suffer from asymptomatic renal and hepatic dysfunction. Care should be taken when administering PREVPAC to this patient population.
ADVERSE REACTIONS
The most common adverse reactions (≥3%) reported in clinical trials when all three components of this therapy were given concomitantly for 14 days are listed in the table below.
| Triple Therapy | |
|---|---|
| n=138 | |
| Adverse Reaction | (%) |
| Diarrhea | 7.0 |
| Headache | 6.0 |
| Taste Perversion | 5.0 |
The additional adverse reactions which were reported as possibly or probably related to treatment (<3%) in clinical trials when all three components of this therapy were given concomitantly are listed below and divided by body system:
Body as a Whole - abdominal pain; Digestive System - dark stools, dry mouth/thirst, glossitis, rectal itching, nausea, oral moniliasis, stomatitis, tongue discoloration, tongue disorder, vomiting; Musculoskeletal System - myalgia; Nervous System - confusion, dizziness; Respiratory System - respiratory disorders; Skin and Appendages - skin reactions; Urogenital System - vaginitis, vaginal moniliasis. There were no statistically significant differences in the frequency of reported adverse events between the 10- and 14-day triple therapy regimens.
PREVACID
The following adverse reactions from the labeling for lansoprazole are provided for information.
Worldwide, over 10,000 patients have been treated with lansoprazole in Phase 2-3 clinical trials involving various dosages and durations of treatment. In general, lansoprazole treatment has been well-tolerated in both short-term and long-term trials.
Incidence in Clinical Trials
The following adverse events were reported by the treating physician to have a possible or probable relationship to drug in 1% or more of PREVACID-treated patients and occurred at a greater rate in PREVACID-treated patients than placebo-treated patients:
| PREVACID | Placebo | |
|---|---|---|
| (N= 2768) | (N= 1023) | |
| Body System/Adverse Event | % | % |
| Body as a Whole | ||
| Abdominal Pain | 2.1 | 1.2 |
| Digestive System | ||
| Constipation | 1.0 | 0.4 |
| Diarrhea | 3.8 | 2.3 |
| Nausea | 1.3 | 1.2 |
Headache was also seen at greater than 1% incidence but was more common on placebo. The incidence of diarrhea was similar between patients who received placebo and patients who received lansoprazole 15 mg and 30 mg, but higher in the patients who received lansoprazole 60 mg (2.9%, 1.4%, 4.2%, and 7.4%, respectively).
The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea.
Additional adverse experiences occurring in <1% of patients or subjects in domestic trials are shown below.
Refer to Postmarketing for adverse reactions occurring since the drug was marketed.
Body as a Whole– abdomen enlarged, allergic reaction, asthenia, back pain, candidiasis, carcinoma, chest pain (not otherwise specified), chills, edema, fever, flu syndrome, halitosis, infection (not otherwise specified), malaise, neck pain, neck rigidity, pain, pelvic pain; Cardiovascular System - angina, arrhythmia, bradycardia, cerebrovascular accident/cerebral infarction, hypertension/hypotension, migraine, myocardial infarction, palpitations, shock (circulatory failure), syncope, tachycardia, vasodilation; Digestive System– abnormal stools, anorexia, bezoar, cardiospasm, cholelithiasis, colitis, dry mouth, dyspepsia, dysphagia, enteritis, eructation, esophageal stenosis, esophageal ulcer, esophagitis, fecal discoloration, flatulence, gastric nodules/fundic gland polyps, gastritis, gastroenteritis, gastrointestinal anomaly, gastrointestinal disorder, gastrointestinal hemorrhage, glossitis, gum hemorrhage, hematemesis, increased appetite, increased salivation, melena, mouth ulceration, nausea and vomiting, nausea and vomiting and diarrhea, oral moniliasis, rectal disorder, rectal hemorrhage, stomatitis, tenesmus, thirst, tongue disorder, ulcerative colitis, ulcerative stomatitis; Endocrine System - diabetes mellitus, goiter, hypothyroidism; Hemic and Lymphatic System - anemia, hemolysis, lymphadenopathy; Metabolic and Nutritional Disorders - gout, dehydration, hyperglycemia/hypoglycemia, peripheral edema, weight gain/loss; Musculoskeletal System - arthralgia, arthritis, bone disorder, joint disorder, leg cramps, musculoskeletal pain, myalgia, myasthenia, synovitis; Nervous System– abnormal dreams, agitation, amnesia, anxiety, apathy, confusion, convulsion, depersonalization, depression, diplopia, dizziness, emotional lability, hallucinations, hemiplegia, hostility aggravated, hyperkinesia, hypertonia, hypesthesia, insomnia, libido decreased/increased, nervousness, neurosis, paresthesia, sleep disorder, somnolence, thinking abnormality, tremor, vertigo; Respiratory System - asthma, bronchitis, cough increased, dyspnea, epistaxis, hemoptysis, hiccup, laryngeal neoplasia, pharyngitis, pleural disorder, pneumonia, respiratory disorder, upper respiratory inflammation/infection, rhinitis, sinusitis, stridor; Skin and Appendages - acne, alopecia, contact dermatitis, dry skin, fixed eruption, hair disorder, maculopapular rash, nail disorder, pruritus, rash, skin carcinoma, skin disorder, sweating, urticaria; Special Senses– abnormal vision, blurred vision, conjunctivitis, deafness, dry eyes, ear disorder, eye pain, otitis media, parosmia, photophobia, retinal degeneration, taste loss, taste perversion, tinnitus, visual field defect; Urogenital System - abnormal menses, breast enlargement, breast pain, breast tenderness, dysmenorrhea, dysuria, gynecomastia, impotence, kidney calculus, kidney pain, leukorrhea, menorrhagia, menstrual disorder, penis disorder, polyuria, testis disorder, urethral pain, urinary frequency, urinary tract infection, urinary urgency, urination impaired, vaginitis.
Postmarketing
On-going Safety Surveillance: Additional adverse experiences have been reported since lansoprazole has been marketed. The majority of these cases are foreign-sourced and a relationship to lansoprazole has not been established. Because these events were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events are listed below by COSTART body system.
Body as a Whole– anaphylactic/anaphylactoid reactions; Digestive System– hepatotoxicity, pancreatitis, vomiting; Hemic and Lymphatic System - agranulocytosis, aplastic anemia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia, and thrombotic thrombocytopenic purpura; Musculoskeletal System– myositis; Skin and Appendages– severe dermatologic reactions including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, (some-fatal); Special Senses– speech disorder; Urogenital System– interstitial nephritis, urinary retention.
Laboratory Values
The following changes in laboratory parameters for lansoprazole were reported as adverse events:
Abnormal liver function tests, increased SGOT (AST), increased SGPT (ALT), increased creatinine, increased alkaline phosphatase, increased globulins, increased GGTP, increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC, bilirubinemia, eosinophilia, hyperlipemia, increased/decreased electrolytes, increased/decreased cholesterol, increased glucocorticoids, increased LDH, increased/decreased/abnormal platelets, and increased gastrin levels. Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported. Additional isolated laboratory abnormalities were reported.
In the placebo-controlled studies, when SGOT (AST) and SGPT (ALT) were evaluated, 0.4% (4/978) placebo patients and 0.4% (11/2677) lansoprazole patients had enzyme elevations greater than three times the upper limit of normal range at the final treatment visit. None of these lansoprazole patients reported jaundice at any time during the study.
Amoxicillin
The following adverse reactions from the labeling for amoxicillin are provided for information.
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillins and in those with a history of allergy, asthma, hay fever, or urticaria.
The following adverse reactions have been reported as associated with the use of penicillins:
Gastrointestinal - Nausea, vomiting, diarrhea, and hemorrhagic/pseudomembranous colitis.
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment (See WARNINGS).
Hypersensitivity Reactions– Serum sickness like reactions, erythematous maculopapular rashes, erythema multiforme, Stevens-Johnson Syndrome, exfoliative dermatitis, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis and urticaria have been reported.
Note: These hypersensitivity reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids. Whenever such reactions occur, amoxicillin should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to amoxicillin therapy.
Liver - A moderate rise in AST (SGOT) and/or ALT (SGPT) has been noted, but the significance of this finding is unknown. Hepatic dysfunction including cholestatic jaundice, hepatic cholestasis and acute cytolytic hepatitis have been reported.
Renal– Crystalluria has also been reported (see OVERDOSAGE).
Hemic and Lymphatic Systems - Anemia, including hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Central Nervous System– Reversible hyperactivity, agitation, anxiety, insomnia, confusion, behavioral changes, and/or dizziness have been reported rarely.
Miscellaneous - Tooth discoloration (brown, yellow, or gray staining) has been rarely reported. Most reports occurred in pediatric patients. Discoloration was reduced or eliminated with brushing or dental cleaning in most cases.
Clarithromycin
The following adverse reactions from the labeling for clarithromycin are provided for information.
The majority of side effects observed in clinical trials were of a mild and transient nature. Fewer than 3% of adult patients without mycobacterial infections discontinued therapy because of drug-related side effects.
The most frequently reported events in adults were diarrhea (3%), nausea (3%), abnormal taste (3%), dyspepsia (2%), abdominal pain/discomfort (2%), and headache (2%). Most of these events were described as mild or moderate in severity. Of the reported adverse events, only 1% was described as severe.
Postmarketing Experience
Allergic reactions ranging from urticaria and mild skin eruptions to rare cases of anaphylaxis, Stevens-Johnson syndrome, and toxic epidermal necrolysis have occurred. Other spontaneously reported adverse events include glossitis, stomatitis, oral moniliasis, anorexia, vomiting, pancreatitis, tongue discoloration, thrombocytopenia, leukopenia, neutropenia, and dizziness. There have been reports of tooth discoloration in patients treated with clarithromycin. Tooth discoloration is usually reversible with professional dental cleaning. There have been isolated reports of hearing loss, which is usually reversible, occurring chiefly in elderly women. Reports of alterations of the sense of smell, usually in conjunction with taste perversion or taste loss have also been reported.
Transient CNS events including anxiety, behavioral changes, confusional states, convulsions, depersonalization, disorientation, hallucinations, insomnia, manic behavior, nightmares, psychosis, tinnitus, tremor, and vertigo have been reported during postmarketing surveillance. Events usually resolve with discontinuation of the drug.
Hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, has been infrequently reported with clarithromycin. This hepatic dysfunction may be severe and is usually reversible. In very rare instances, hepatic failure with fatal outcome has been reported and generally has been associated with serious underlying diseases and/or concomitant medications.
There have been rare reports of hypoglycemia, some of which have occurred in patients taking oral hypoglycemic agents or insulin.
As with other macrolides, clarithromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes.
There have been reports of interstitial nephritis coincident with clarithromycin use.
There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients. (See WARNINGS and PRECAUTIONS).
Changes in Laboratory Values
Changes in laboratory values with possible clinical significance were as follows: Hepatic - elevated SGPT (ALT) < 1%, SGOT (AST) < 1%, GGT < 1%, alkaline phosphatase < 1%, LDH < 1%, total bilirubin < 1%; Hematologic - decreased WBC < 1%, elevated prothrombin time 1%; Renal - elevated BUN 4%, elevated serum creatinine < 1%. GGT, alkaline phosphatase, and prothrombin time data are from adult studies only.
OVERDOSAGE
In case of an overdose, patients should contact a physician, poison control center, or emergency room. There is neither a pharmacologic basis nor data suggesting an increased toxicity of the combination compared to individual components.
Lansoprazole
Oral doses up to 5000 mg/kg in rats (approximately 1300 times the 30 mg human dose based on body surface area) and mice (about 675.7 times the 30 mg human dose based on body surface area) did not produce deaths or any clinical signs.
Lansoprazole is not removed from the circulation by hemodialysis. In one reported case of overdose, the patient consumed 600 mg of lansoprazole with no adverse reaction.
Amoxicillin
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures as required. If the overdosage is very recent and there is no contraindication, an attempt at emesis or other means of removal of drug from the stomach may be performed. A prospective study of 51 pediatric patients at a poison-control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms and do not require gastric emptying2.
Interstitial nephritis resulting in oliguric renal failure has been reported in a small number of patients after overdosage with amoxicillin.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage in adult and pediatric patients. In case of overdosage, adequate fluid intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria. Renal impairment appears to be reversible with cessation of drug administration. High blood levels may occur more readily in patients with impaired renal function because of decreased renal clearance of amoxicillin. Amoxicillin can be removed from circulation by hemodialysis.
Clarithromycin
Overdosage of clarithromycin can cause gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea.
Adverse reactions accompanying overdosage should be treated by the prompt elimination of unabsorbed drug and supportive measures. As with other macrolides, clarithromycin serum levels are not expected to be appreciably affected by hemodialysis or peritoneal dialysis.
DOSAGE AND ADMINISTRATION
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
The recommended adult oral dose is 30 mg PREVACID, 1 g amoxicillin, and 500 mg clarithromycin administered together twice daily (morning and evening) for 10 or 14 days. (See INDICATIONS AND USAGE).
PREVPAC is not recommended in patients with creatinine clearance less than 30 mL/min.
HOW SUPPLIED
PREVPAC is supplied as an individual daily administration pack, each containing:
PREVACID:
- –
- two opaque, hard gelatin, black and pink PREVACID 30-mg capsules, with the TAP logo and "PREVACID 30" imprinted on the capsules.
Amoxicillin Capsules, USP:
- –
- four yellow, opaque, hard gelatin amoxicillin 500-mg capsules, USP, imprinted AMOX 500 on one side and GG 849 on the other side.
BIAXIN Filmtab:
- –
- two yellow oval film-coated clarithromycin 500-mg tablets, USP, debossed with the Abbott logo on one side and "KL" on the other side of the tablets.
NDC 0300-3702-01 Daily administration pack
NDC 0300-3702-11 Daily administration card
Store at a controlled room temperature between 20°C and 25°C (68°F and 77°F). Protect from light and moisture.
Rx only
REFERENCE
- National Committee for Clinical Laboratory Standards. Summary Minutes, Subcommittee on Antimicrobial Susceptibility Testing, Tampa, FL, January 11-13, 1998.
- Swanson Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988; 30:66-67.
U.S. Patent No. 5,013,743
PREVPAC is distributed by TAP Pharmaceuticals Inc.
PREVACID® (lansoprazole) Delayed-Release Capsules
Distributed by TAP Pharmaceuticals Inc.
Lake Forest, IL 60045, U.S.A.
Amoxicillin Capsules, USP
Manufactured by Sandoz GmbH, Kundl, Austria
for Sandoz Inc., Broomfield, CO 80020, U.S.A.
BIAXIN® Filmtab® (clarithromycin tablets, USP)
Manufactured by Abbott Laboratories
North Chicago, IL 60064, U.S.A.
03-5529-R9, Rev. March 2007
©1997-2007 TAP Pharmaceutical Products Inc.
(No. 3702)
| PREVPAC (lansoprazole, amoxicillin and clarithromycin) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revised: 06/2007