RECOMBIVAX HB
-
hepatitis b virus subtype adw hbsag surface protein antigen injection, suspension
Merck Sharp & Dohme Corp.
----------
RECOMBIVAX HB®HEPATITIS B VACCINE (RECOMBINANT)
DESCRIPTION
RECOMBIVAX HB® Hepatitis B Vaccine (Recombinant) is a non-infectious subunit viral vaccine derived from hepatitis B surface antigen (HBsAg) produced in yeast cells. A portion of the hepatitis B virus gene, coding for HBsAg, is cloned into yeast, and the vaccine for hepatitis B is produced from cultures of this recombinant yeast strain according to methods developed in the Merck Research Laboratories.
The antigen is harvested and purified from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg. The fermentation process involves growth of Saccharomyces cerevisiae on a complex fermentation medium which consists of an extract of yeast, soy peptone, dextrose, amino acids and mineral salts. The HBsAg protein is released from the yeast cells by cell disruption and purified by a series of physical and chemical methods. The purified protein is treated in phosphate buffer with formaldehyde and then coprecipitated with alum (potassium aluminum sulfate) to form bulk vaccine adjuvanted with amorphous aluminum hydroxyphosphate sulfate. The vaccine contains no detectable yeast DNA but may contain not more than 1% yeast protein. The vaccine produced by the Merck method has been shown to be comparable to the plasma-derived vaccine in terms of animal potency (mouse, monkey, and chimpanzee) and protective efficacy (chimpanzee and human).
The vaccine against hepatitis B, prepared from recombinant yeast cultures, is free of association with human blood or blood products.
Each lot of hepatitis B vaccine is tested for sterility.
RECOMBIVAX HB is a sterile suspension for intramuscular injection. However, for persons at risk of hemorrhage following intramuscular injection, the vaccine may be administered subcutaneously. (See DOSAGE AND ADMINISTRATION).
RECOMBIVAX HB Hepatitis B Vaccine (Recombinant) is supplied in three formulations. (See HOW SUPPLIED.)
Pediatric/Adolescent Formulation (Without Preservative), 10 mcg/mL: each 0.5 mL dose contains 5 mcg of hepatitis B surface antigen.
Adult Formulation (Without Preservative), 10 mcg/mL: each 1 mL dose contains 10 mcg of hepatitis B surface antigen.
Dialysis Formulation (Without Preservative), 40 mcg/mL: each 1 mL dose contains 40 mcg of hepatitis B surface antigen.
All formulations contain approximately 0.5 mg of aluminum (provided as amorphous aluminum hydroxyphosphate sulfate, previously referred to as aluminum hydroxide) per mL of vaccine. In each formulation, hepatitis B surface antigen is adsorbed onto approximately 0.5 mg of aluminum (provided as amorphous aluminum hydroxyphosphate sulfate) per mL of vaccine. The vaccine contains <15 mcg/mL residual formaldehyde. The vaccine is of the adw subtype. RECOMBIVAX HB is indicated for vaccination of persons at risk of infection from hepatitis B virus including all known subtypes. RECOMBIVAX HB Dialysis Formulation is indicated for vaccination of adult predialysis and dialysis patients against infection caused by all known subtypes of hepatitis B virus.
CLINICAL PHARMACOLOGY
Hepatitis B virus is one of several hepatitis viruses that cause a systemic infection, with a major pathology in the liver. These include hepatitis A virus, hepatitis D virus, and hepatitis C and E viruses, previously referred to as non-A, non-B hepatitis viruses.
Hepatitis B virus is an important cause of viral hepatitis. There is no specific treatment for this disease. The incubation period for hepatitis B is relatively long; six weeks to six months may elapse between exposure and the onset of clinical symptoms. The prognosis following infection with hepatitis B virus is variable and dependent on at least three factors: (1) Age — Infants and younger children usually experience milder initial disease than older persons;{1} (2) Dose of virus — The higher the dose, the more likely acute icteric hepatitis B will result;{1} and, (3) Severity of associated underlying disease — Underlying malignancy or pre-existing hepatic disease predisposes to increased morbidity and mortality.{1}
Persistence of viral infection (the chronic hepatitis B virus carrier state) occurs in 5-10% of persons following acute hepatitis B, and occurs more frequently after initial anicteric hepatitis B than after initial icteric disease. Consequently, carriers of hepatitis B surface antigen (HBsAg) frequently give no history of having had recognized acute hepatitis. The Centers for Disease Control and Prevention (CDC) estimates that there are more than 300 million chronic carriers worldwide and 1.25 million chronic carriers of hepatitis B virus in the USA.{29,30} Chronic carriers represent the largest human reservoir of hepatitis B virus.
Serious complications and sequelae of hepatitis B virus infection include massive hepatic necrosis, cirrhosis of the liver and chronic active hepatitis. More than one million people worldwide die each year of hepatitis B-associated acute and chronic liver disease.{33} In the United States, hepatitis B-virus-related acute and chronic liver disease causes approximately 4-5000 deaths annually.{29,30}
Reduced Risk of Hepatocellular Carcinoma
Hepatocellular carcinoma is another serious complication of hepatitis B virus infection. Studies have demonstrated the link between chronic hepatitis B infection and hepatocellular carcinoma; 80% of primary liver cancers are caused by hepatitis B virus infection. The CDC has recognized hepatitis B vaccine as the first anti-cancer vaccine because it can prevent primary liver cancer.{34}
There is also evidence that several diseases other than hepatitis have been associated with hepatitis B virus infection through an immunologic mechanism involving antigen-antibody complexes. Such diseases include a syndrome with rash, urticaria, and arthralgia resembling serum sickness; periarteritis nodosa; membranous glomerulonephritis; and infantile papular acrodermatitis.{3,4}
Although the vehicles for transmission of the virus are often blood and blood products, viral antigen has also been found in tears, saliva, breast milk, urine, semen and vaginal secretions. Hepatitis B virus is capable of surviving at least a month{29} on environmental surfaces exposed to body fluids containing hepatitis B virus. Infection may occur when hepatitis B virus, transmitted by infected body fluids, is implanted via mucous surfaces or percutaneously introduced through accidental or deliberate breaks in the skin.
Transmission of hepatitis B virus infection is often associated with close interpersonal contact with an infected individual and with crowded living conditions. In such circumstances, transmission by inoculation via routes other than overt percutaneous ones may be quite common.{1} Perinatal transmission of hepatitis B infection from infected mother to child, at or shortly after birth, can occur if the mother is a hepatitis B surface antigen (HBsAg) carrier or if the mother has an acute hepatitis B infection in the third trimester. Infection in infancy by the hepatitis B virus usually leads to the chronic carrier state. Without prophylaxis, infants born to women whose sera are positive for both the hepatitis B surface antigen and the e antigen have an 85-90% likelihood of being infected and becoming a chronic carrier.{5,6} Well-controlled studies have shown that administration of three 0.5 mL doses of Hepatitis B Immune Globulin (Human) - HBIG starting at birth is 75% effective in preventing establishment of the chronic carrier state in these infants during the first year of life.{6} However, the protective effect of HBIG is transient.
Hepatitis B is endemic throughout the world and is a serious medical problem in population groups at increased risk. Because vaccination limited to high-risk individuals has failed to substantially lower the overall incidence of hepatitis B infection, both the Advisory Committee on Immunization Practices (ACIP) and the Committee on Infectious Diseases of the American Academy of Pediatrics (AAP) have also endorsed universal infant immunization as part of a comprehensive strategy for the control of hepatitis B infection.{7,8} In addition, the ACIP also recommends hepatitis B vaccination for all infants and children born after November 21, 1991 and catch-up vaccination of children at high risk of infection (children <11 years of age in households of Pacific Islander ethnicity or of first generation immigrants/refugees from countries with an intermediate or high endemicity of infection).{30} These advisory groups further recommend broad-based vaccination of adolescents. The ACIP recommends that all individuals not previously vaccinated with hepatitis B vaccine be vaccinated at 11-12 years of age with the age-appropriate dose of vaccine and that the vaccination schedule take into account the feasibility of delivering three doses of vaccine to this age group. In addition, older unvaccinated adolescents with identified risk factors for hepatitis B virus infection should also be vaccinated.{30} Similarly, the AAP recommends that universal immunization of all adolescents should be implemented when resources permit with emphasis on those individuals in high-risk settings.{8} A National Institutes of Health Consensus Development Conference Panel on the management of hepatitis C recommends the immunization of all hepatitis C virus (HCV) positive individuals with hepatitis B vaccine.{35} (Refer to INDICATIONS AND USAGE.)
Numerous epidemiological studies have shown that persons who develop anti-HBs following active infection with the hepatitis B virus are protected against the disease on re-exposure to the virus.{9}
Clinical studies have shown that RECOMBIVAX HB when injected into the deltoid muscle induced protective levels of antibody in 96% of 1213 healthy adults who received the recommended 3-dose regimen. Antibody responses varied with age; a protective level of antibody was induced in 98% of 787 young adults 20-29 years of age, 94% of 249 adults 30-39 years of age and in 89% of 177 adults ≥40 years of age.{10} Studies with hepatitis B vaccine derived from plasma have shown that a lower response rate (81%) to vaccine may be obtained if the vaccine is administered as a buttock injection.{11} Seroconversion rates and geometric mean antibody titers were measured 1 to 2 months after the third dose. Multiple clinical studies have defined a protective antibody (anti-HBs) level as 1) 10 or more sample ratio units (SRU) as determined by radioimmunoassay or 2) a positive result as determined by enzyme immunoassay.{2} Note: 10 SRU is comparable to 10 mIU/mL of antibody.{12,13,14,15}
RECOMBIVAX HB was shown to be highly immunogenic in clinical studies involving infants, children, and adolescents. Three 5 mcg doses of vaccine induced a protective level of antibody in 100% of 92 infants, 99% of 129 children, and in 99% of 112 adolescents{10} (see DOSAGE AND ADMINISTRATION).
The protective efficacy of three 5 mcg doses of RECOMBIVAX HB has been demonstrated in neonates born of mothers positive for both HBsAg and HBeAg (a core-associated antigenic complex which correlates with high infectivity). In a clinical study of infants who received one dose of HBIG at birth followed by the recommended three-dose regimen of RECOMBIVAX HB, chronic infection had not occurred in 96% of 130 infants after nine months of follow-up.{16} The estimated efficacy in prevention of chronic hepatitis B infection was 95% as compared to the infection rate in untreated historical controls.{17} Significantly fewer neonates became chronically infected when given one dose of HBIG at birth followed by the recommended three-dose regimen of RECOMBIVAX HB when compared to historical controls who received only a single dose of HBIG.{6} Testing for HBsAg and anti-HBs is recommended at 12-15 months of age. If HBsAg is not detectable, and anti-HBs is present, the child has been protected.
As demonstrated in the above study, HBIG, when administered simultaneously with RECOMBIVAX HB at separate body sites, did not interfere with the induction of protective antibodies against hepatitis B virus elicited by the vaccine.
For adolescents (11 through 15 years of age), the immunogenicity of a two-dose regimen (10 mcg at 0 and 4-6 months) was compared with that of the standard three-dose regimen (5 mcg at 0, 1, and 6 months) in an open, randomized, multicenter study. The proportion of adolescents receiving the two-dose regimen who developed a protective level of antibody one month after the last dose (99% of 255 subjects) appears similar to that among adolescents who received the three-dose regimen (98% of 121 subjects). After adolescents (11 through 15 years of age) received the first 10-mcg dose of the two-dose regimen, the proportion who developed a protective level of antibody was approximately 72%.{10}
In one published study, the seroprotection rates in individuals with chronic HCV infection given the standard regimen of RECOMBIVAX HB was approximately 70%.{36} In a second published study of intravenous drug users given an accelerated schedule of RECOMBIVAX HB, infection with HCV did not affect the response to RECOMBIVAX HB.{37}
As with other hepatitis B vaccines, the duration of the protective effect of RECOMBIVAX HB in healthy vaccinees is unknown at present, and the need for booster doses is not yet defined. However, long-term follow-up (5 to 9 years) of approximately 3000 high-risk vaccinees (infants of carrier mothers, male homosexuals, Alaskan Natives) who developed an anti-HBs titer of ≥10 mIU/mL when given a similar plasma-derived vaccine at intervals of 0, 1, and 6 months showed that no subjects developed clinically apparent hepatitis B infection and that 5 subjects developed antigenemia, even though up to half of the subjects failed to maintain a titer at this level.{18-21} Persistence of vaccine-induced immunologic memory among healthy vaccinees who responded to a primary course of plasma-derived or recombinant hepatitis B vaccine has been demonstrated by an anamnestic antibody response to a booster dose of RECOMBIVAX HB given 5-12 years later.{22}
Predialysis and Dialysis Patients
Predialysis and dialysis adult patients respond less well to hepatitis B vaccines than do healthy individuals; however, vaccination of adult patients early in the course of their renal disease produces higher seroconversion rates than vaccination after dialysis has been initiated.{30} In addition, the responses to these vaccines may be lower if the vaccine is administered as a buttock injection. When 40 mcg of Hepatitis B Vaccine (Recombinant), was administered in the deltoid muscle, 89% of 28 participants developed anti-HBs with 86% achieving levels ≥10 mIU/mL. However, when the same dosage of this vaccine was administered inappropriately either in the buttock or a combination of buttock and deltoid, 62% of 47 participants developed anti-HBs with 55% achieving levels of ≥10 mIU/mL.{10}
A booster dose or revaccination with RECOMBIVAX HB Dialysis Formulation may be considered in predialysis/dialysis patients if the anti-HBs level is less than 10 mIU/mL.{23}
Reports in the literature describe a more virulent form of hepatitis B associated with superinfections or coinfections by delta virus, an incomplete RNA virus. Delta virus can only infect and cause illness in persons infected with hepatitis B virus since the delta agent requires a coat of HBsAg in order to become infectious. Therefore, persons immune to hepatitis B virus infection should also be immune to delta virus infection.{2}
Interchangeability of Plasma-Derived and Recombinant Hepatitis B Vaccines
Although there have been no clinical studies in which a three-dose vaccine series was initiated with HEPTAVAX-B® (Hepatitis B Vaccine) and completed with RECOMBIVAX HB, or vice versa, extensive in vitro and in vivo studies have demonstrated that these two vaccines are immunologically comparable.{22,24-28}
INDICATIONS AND USAGE
RECOMBIVAX HB is indicated for vaccination against infection caused by all known subtypes of hepatitis B virus. RECOMBIVAX HB Dialysis Formulation is indicated for vaccination of adult predialysis and dialysis patients against infection caused by all known subtypes of hepatitis B virus.
Vaccination with RECOMBIVAX HB is recommended for:
- Infants including those born to HBsAg positive mothers (high-risk infants).
- Children born after November 21, 1991.{30}
- Adolescents (see CLINICAL PHARMACOLOGY).
- Other persons of all ages in areas of high prevalence or those who are or may be at increased risk of infection with hepatitis B virus, such as:{30}
-
Health Care Personnel
Dentists and oral surgeons.
Physicians and surgeons.
Nurses.
Paramedical personnel and custodial staff who may be exposed to the virus via blood or other patient specimens.
Dental hygienists and dental nurses.
Laboratory personnel handling blood, blood products, and other patient specimens.
Dental, medical and nursing students. -
Selected Patients and Patient Contacts
Staff in hemodialysis units and hematology/oncology units.
Hemodialysis patients and patients with early renal failure before they require hemodialysis.
Patients requiring frequent and/or large volume blood transfusions or clotting factor concentrates (e.g., persons with hemophilia, thalassemia).
Individuals with hepatitis C virus infection.{35}
Clients (residents) and staff of institutions for the mentally handicapped.
Classroom contacts of deinstitutionalized mentally handicapped persons who have persistent hepatitis B surface antigenemia and who show aggressive behavior.
Household and other intimate contacts of persons with persistent hepatitis B surface antigenemia. -
Sub-populations with a known high incidence of the disease, such as:
Alaskan Natives.
Pacific Islanders.
Refugees from areas where hepatitis B virus infection is endemic.
Adoptees from countries where hepatitis B virus infection is endemic. - International Travelers
- Military Personnel identified as being at increased risk
- Morticians and Embalmers
- Blood bank and plasma fractionation workers
-
Persons at Increased Risk of the Disease Due to Their Sexual Practices, such as:
Persons who have heterosexual activity with multiple partners.
Persons who repeatedly contract sexually transmitted diseases.
Homosexual and bisexual adolescent and adult men.
Female prostitutes. - Prisoners
- Injection drug users
Neither dosage strength will prevent hepatitis caused by other agents, such as hepatitis A virus, hepatitis C virus, hepatitis E virus or other viruses known to infect the liver.
Revaccination
Use with Other Vaccines
Results from clinical studies indicate that RECOMBIVAX HB can be administered concomitantly with DTP (Diphtheria, Tetanus and whole cell Pertussis), OPV (oral Poliomyelitis vaccine), M-M-R® II (Measles, Mumps, and Rubella Virus Vaccine Live), Liquid PedvaxHIB® [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)] or a booster dose of DTaP [Diphtheria, Tetanus, acellular Pertussis], using separate sites and syringes for injectable vaccines. No impairment of immune response to individually tested vaccine antigens was demonstrated.
The type, frequency and severity of adverse experiences observed in these studies with RECOMBIVAX HB were similar to those seen when the other vaccines were given alone.
In addition, an HBsAg-containing product, COMVAX® [Haemophilus b Conjugate (Meningococcal Protein Conjugate) and Hepatitis B (Recombinant) Vaccine], was given concomitantly with eIPV (enhanced inactivated Poliovirus vaccine) or VARIVAX® [Varicella Virus Vaccine Live (Oka/Merck)], using separate sites and syringes for injectable vaccines. No impairment of immune response to these individually tested vaccine antigens was demonstrated. No serious vaccine-related adverse events were reported.
COMVAX has also been administered concomitantly with the primary series of DTaP to a limited number of infants. No serious vaccine-related adverse events were reported.{10}
Separate sites and syringes should be used for simultaneous administration of injectable vaccines.
CONTRAINDICATIONS
Hypersensitivity to yeast or any component of the vaccine.
WARNINGS
Patients who develop symptoms suggestive of hypersensitivity after an injection should not receive further injections of the vaccine (see CONTRAINDICATIONS).
Because of the long incubation period for hepatitis B, it is possible for unrecognized infection to be present at the time the vaccine is given. The vaccine may not prevent hepatitis B in such patients.
PRECAUTIONS
General
As with any percutaneous vaccine, epinephrine (1:1000) should be available for immediate use should an anaphylactoid reaction occur.
Use caution when vaccinating latex-sensitive individuals since the vial stopper and the syringe plunger stopper and tip cap contain dry natural latex rubber that may cause allergic reactions.
Any serious active infection including febrile illness is reason for delaying use of the vaccine except when in the opinion of the physician, withholding the vaccine entails a greater risk.
Caution and appropriate care should be exercised in administering the vaccine to individuals with severely compromised cardiopulmonary status or to others in whom a febrile or systemic reaction could pose a significant risk.
Instructions to Healthcare Provider
The healthcare provider should determine the current health status and previous vaccination history of the vaccinee.
The healthcare provider should question the patient, parent or guardian about reactions to a previous dose of RECOMBIVAX HB or other hepatitis B vaccines.
The healthcare provider must record in the patient’s permanent record: the manufacturer, lot number, date of administration, and the name and address of the person administering the vaccine.
Injection of a blood vessel should be avoided.
Information for Vaccine Recipients and Parents/Guardians
The healthcare provider should provide the vaccine information required to be given with each vaccination to the patient, parent or guardian.
The healthcare provider should inform the patient, parent or guardian of the benefits and risks associated with vaccination, as well as the importance of completing the immunization series. For risks associated with vaccination, see WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS.
Patients, parents and guardians should be instructed to report any serious adverse reactions to their healthcare provider, who in turn should report such events to the U.S. Department of Health and Human Services through the Vaccine Adverse Event Reporting System (VAERS), 1-800-822-7967.{31} The healthcare provider should inform the parent or guardian of the National Vaccine Injury Compensation Program (NVICP), 1-800-338-2382.
Drug Interactions
There are no known drug interactions. (See INDICATIONS AND USAGE, Use with Other Vaccines.)
Carcinogenesis, Mutagenesis, Impairment of Fertility
RECOMBIVAX HB has not been evaluated for its carcinogenic or mutagenic potential, or its potential to impair fertility.
Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with the vaccine. It is also not known whether the vaccine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. The vaccine should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether the vaccine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when the vaccine is administered to a nursing woman.
Pediatric Use
RECOMBIVAX HB has been shown to be usually well-tolerated and highly immunogenic in infants and children of all ages. Newborns also respond well; maternally transferred antibodies do not interfere with the active immune response to the vaccine. See DOSAGE AND ADMINISTRATION for recommended pediatric dosage and for recommended dosage for infants born to HBsAg positive mothers.
The safety and effectiveness of RECOMBIVAX HB Dialysis Formulation in children have not been established.
Geriatric Use
Clinical studies of RECOMBIVAX HB did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reports from the clinical literature indicate that hepatitis B vaccines are less immunogenic in adults aged 65 years or older than in younger individuals.{32} No overall differences in safety were observed between these subjects and younger subjects.
ADVERSE REACTIONS
RECOMBIVAX HB and RECOMBIVAX HB Dialysis Formulation are generally well-tolerated. No adverse experiences were reported during clinical trials which could be related to changes in the titers of antibodies to yeast. As with any vaccine, there is the possibility that broad use of the vaccine could reveal adverse reactions not observed in clinical trials.
In three clinical studies, 434 doses of RECOMBIVAX HB, 5 mcg, were administered to 147 healthy infants and children (up to 10 years of age) who were monitored for 5 days after each dose. Injection site reactions and systemic complaints were reported following 0.2% and 10.4% of the injections, respectively. The most frequently reported systemic adverse reactions (>1% injections), in decreasing order of frequency, were irritability, fever (≥101°F oral equivalent), diarrhea, fatigue/weakness, diminished appetite, and rhinitis.{10}
In a study that compared the three-dose regimen (5 mcg) with the two-dose regimen (10 mcg) of RECOMBIVAX HB in adolescents, the overall frequency of adverse reactions was generally similar.
In a group of studies, 3258 doses of RECOMBIVAX HB, 10 mcg, were administered to 1252 healthy adults who were monitored for 5 days after each dose. Injection site reactions and systemic complaints were reported following 17% and 15% of the injections, respectively. The following adverse reactions were reported:
Incidence Equal To or Greater Than 1% of Injections
LOCAL REACTION (INJECTION SITE)
Injection site reactions consisting principally of soreness, and including pain, tenderness, pruritus, erythema, ecchymosis, swelling, warmth, and nodule formation.
BODY AS A WHOLE
The most frequent systemic complaints include fatigue/weakness; headache; fever (≥100°F); and malaise.
DIGESTIVE SYSTEM
Nausea; and diarrhea
RESPIRATORY SYSTEM
Pharyngitis; and upper respiratory infection
Incidence Less Than 1% of Injections
BODY AS A WHOLE
Sweating; achiness; sensation of warmth; lightheadedness; chills; and flushing
DIGESTIVE SYSTEM
Vomiting; abdominal pains/cramps; dyspepsia; and diminished appetite
RESPIRATORY SYSTEM
Rhinitis; influenza; and cough
NERVOUS SYSTEM
Vertigo/dizziness; and paresthesia
INTEGUMENTARY SYSTEM
Pruritus; rash (non-specified); angioedema; and urticaria
MUSCULOSKELETAL SYSTEM
Arthralgia including monoarticular; myalgia; back pain; neck pain; shoulder pain; and neck stiffness
HEMIC/LYMPHATIC SYSTEM
Lymphadenopathy
PSYCHIATRIC/BEHAVIORAL
Insomnia/disturbed sleep
SPECIAL SENSES
Earache
UROGENITAL SYSTEM
Dysuria
CARDIOVASCULAR SYSTEM
Hypotension
Marketed Experience
The following additional adverse reactions have been reported with use of the marketed vaccine. In many instances, the relationship to the vaccine was unclear.
Hypersensitivity
Anaphylaxis and symptoms of immediate hypersensitivity reactions including rash, pruritus, urticaria, edema, angioedema, dyspnea, chest discomfort, bronchial spasm, palpitation, or symptoms consistent with a hypotensive episode have been reported within the first few hours after vaccination. An apparent hypersensitivity syndrome (serum-sickness-like) of delayed onset has been reported days to weeks after vaccination, including: arthralgia/arthritis (usually transient), fever, and dermatologic reactions such as urticaria, erythema multiforme, ecchymoses and erythema nodosum (see WARNINGS and PRECAUTIONS).
Digestive System
Elevation of liver enzymes; constipation
Nervous System
Guillain-Barré Syndrome; multiple sclerosis; exacerbation of multiple sclerosis; myelitis including transverse myelitis; seizure; febrile seizure; peripheral neuropathy including Bell's Palsy; radiculopathy; herpes zoster; migraine; muscle weakness; hypesthesia; encephalitis
Integumentary System
Stevens-Johnson Syndrome; alopecia; petechiae; eczema
Musculoskeletal System
Arthritis
Pain in extremity
Hematologic
Increased erythrocyte sedimentation rate; thrombocytopenia
Immune System
Systemic lupus erythematosus (SLE); lupus-like syndrome; vasculitis; polyarteritis nodosa
Psychiatric/Behavioral
Irritability; agitation; somnolence
Special Senses
Optic neuritis; tinnitus; conjunctivitis; visual disturbances; uveitis
Cardiovascular System
Syncope; tachycardia.
The following adverse reaction has been reported with another Hepatitis B Vaccine (Recombinant) but not with RECOMBIVAX HB: keratitis.
Patients, parents and guardians should be instructed to report any serious adverse reactions to their healthcare provider, who in turn should report such events to the U.S. Department of Health and Human Services through the Vaccine Adverse Event Reporting System (VAERS), 1-800-822-7967.{31}
DOSAGE AND ADMINISTRATION
Do not inject intravenously or intradermally.
RECOMBIVAX HB Hepatitis B Vaccine (Recombinant) DIALYSIS FORMULATION [(40 mcg/mL) (WITHOUT PRESERVATIVE)] IS INTENDED ONLY FOR ADULT PREDIALYSIS/DIALYSIS PATIENTS.
RECOMBIVAX HB Hepatitis B Vaccine (Recombinant) PEDIATRIC/ADOLESCENT (WITHOUT PRESERVATIVE) and ADULT FORMULATIONS (WITHOUT PRESERVATIVE) ARE NOT INTENDED FOR USE IN PREDIALYSIS/DIALYSIS PATIENTS.
Three-Dose Regimen
The vaccination regimen for each population consists of 3 doses of vaccine given according to the following schedule:
First dose: at elected date
Second dose: 1 month later
Third dose: 6 months after the first dose
For infants born of mothers who are HBsAg positive or mothers of unknown HBsAg status, treatment recommendations are described in the subsection titled: Guidelines for Treatment of Infants Born of HBsAg Positive Mothers or Mothers of Unknown HBsAg Status.
Two-Dose Regimen – Adolescents (11 through 15 years of age)
An alternate two-dose regimen is available for routine vaccination of adolescents (11 through 15 years of age). The regimen consists of two doses of vaccine (10 mcg) given according to the following schedule:
First injection: at elected date
Second injection: 4-6 months later
Table 1 summarizes the dose and formulation of RECOMBIVAX HB for specific populations, regardless of the risk of infection with hepatitis B virus.
| Group | Dose/Regimen | Formulation | Color Code |
|
|||
| Infants, Children and Adolescents 0-19 years of age | 5 mcg (0.5 mL) 3 x 5 mcg | Pediatric/Adolescent | Yellow |
| Adolescents*
11 through 15 years of age | 10 mcg† (1.0 mL) 2 x 10 mcg | Adult | Green |
| Adults ≥20 years of age | 10 mcg† (1.0 mL) 3 x 10 mcg | Adult | Green |
| Predialysis and Dialysis Patients‡ | 40 mcg (1.0 mL) 3 x 40 mcg | Dialysis | Blue |
RECOMBIVAX HB is for intramuscular injection. The deltoid muscle is the preferred site for intramuscular injection in adults. Data suggest that injections given in the buttocks frequently are given into fatty tissue instead of into muscle. Such injections have resulted in a lower seroconversion rate than was expected. The anterolateral thigh is the recommended site for intramuscular injection in infants and young children.
For persons at risk of hemorrhage following intramuscular injection, RECOMBIVAX HB may be administered subcutaneously. However, when other aluminum-adsorbed vaccines have been administered subcutaneously, an increased incidence of local reactions including subcutaneous nodules has been observed. Therefore, subcutaneous administration should be used only in persons (e.g., hemophiliacs) who are at risk of hemorrhage following intramuscular injections.
The vaccine should be used as supplied; no dilution or reconstitution is necessary. The full recommended dose of the vaccine should be used.
For All Formulations: Since none of the formulations contain a preservative, once the single-dose vial has been penetrated, the withdrawn vaccine should be used promptly, and the vial must be discarded.
Shake well before use. Thorough agitation at the time of administration is necessary to maintain suspension of the vaccine.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. After thorough agitation, the vaccine is a slightly opaque, white suspension.
Withdraw the recommended dose from the vial using a sterile needle and syringe free of preservatives, antiseptics, and detergents.
It is important to use a separate sterile syringe and needle for each individual patient to prevent transmission of hepatitis and other infectious agents from one person to another. Needles should be disposed of properly and should not be recapped.
Injection must be accomplished with a needle long enough to ensure intramuscular deposition of the vaccine.
Guidelines for Treatment of Infants Born of HBsAg Positive Mothers or Mothers of Unknown HBsAg Status
Each infant should receive three 5 mcg doses of RECOMBIVAX HB irrespective of the mother’s HBsAg status (see Table 1). The ACIP recommends that if the mother is determined to be HBsAg positive within 7 days of delivery, the infant also should be given a dose of HBIG (0.5 mL) immediately. The first dose of RECOMBIVAX HB may be given at the same time as HBIG, but it should be administered in the opposite anterolateral thigh.{7}
Revaccination
The duration of the protective effect of RECOMBIVAX HB in healthy vaccinees is unknown at present and the need for booster doses is not yet defined (see CLINICAL PHARMACOLOGY).
A booster dose or revaccination with RECOMBIVAX HB Dialysis Formulation (blue color code) may be considered in predialysis/dialysis patients if the anti-HBs level is less than 10 mIU/mL 1 to 2 months after the third dose.{23} The ACIP recommends that the need for booster doses of vaccine should be assessed by annual antibody testing and a booster dose given when antibody levels decline to <10 mIU/mL.{30}
Known or Presumed Exposure to HBsAg
There are no prospective studies directly testing the efficacy of a combination of HBIG and RECOMBIVAX HB in preventing clinical hepatitis B following percutaneous, ocular or mucous membrane exposure to hepatitis B virus. However, since most persons with such exposures (e.g., health-care workers) are candidates for RECOMBIVAX HB and since combined HBIG plus vaccine is more efficacious than HBIG alone in perinatal exposures, the following guidelines are recommended for persons who have been exposed to hepatitis B virus such as through (1) percutaneous (needlestick), ocular, mucous membrane exposure to blood known or presumed to contain HBsAg, (2) human bites by known or presumed HBsAg carriers, that penetrate the skin, or (3) following intimate sexual contact with known or presumed HBsAg carriers.
HBIG (0.06 mL/kg) should be given intramuscularly as soon as possible after exposure and within 24 hours if possible. RECOMBIVAX HB (see dosage recommendation) should be given intramuscularly at a separate site within 7 days of exposure and second and third doses given one and six months, respectively, after the first dose.
Prefilled Syringe
Shake well before use. Attach the needle by twisting in a clockwise direction until the needle fits securely on the syringe. Administer the entire dose as per standard protocol.
HOW SUPPLIED
PEDIATRIC/ADOLESCENT FORMULATION (PRESERVATIVE FREE)
Vials
No. 4980 — RECOMBIVAX HB for use in infants, children, and adolescents is supplied as 5 mcg/0.5 mL of HBsAg in a 0.5 mL single-dose vial, color coded with a yellow cap and stripe on the vial labels and cartons and an orange banner on the vial labels and cartons stating “Preservative Free”, NDC 0006-4980-00.
No. 4981 — RECOMBIVAX HB for use in infants, children, and adolescents is supplied as 5 mcg/0.5 mL of HBsAg in a 0.5 mL single-dose vial, in a box of 10 single-dose vials, color coded with a yellow cap and stripe on the vial labels and cartons and an orange banner on the vial labels and cartons stating “Preservative Free”, NDC 0006-4981-00.
Syringes
No. 4093 — RECOMBIVAX HB for use in infants, children and adolescents is supplied as 5 mcg/0.5 mL of HBsAg in a carton of 6 prefilled single-dose Luer Lock syringes with tip caps, color coded with a yellow plunger rod and stripe on the peel-off syringe labels and cartons and an orange banner on the cartons stating “Preservative Free”, NDC 0006-4093-09.
ADULT FORMULATION (PRESERVATIVE FREE)
Vials
No. 4995 — RECOMBIVAX HB for use in adults and adolescents (11 through 15 years of age) is supplied as 10 mcg/mL of HBsAg in a 1 mL single-dose vial, color coded with a green cap and stripe on the vial labels and cartons and an orange banner on the vial labels and cartons stating “Preservative Free”, NDC 0006-4995-00.
No. 4995 — RECOMBIVAX HB for use in adults and adolescents (11 through 15 years of age) is supplied as 10 mcg/mL of HBsAg in a 1 mL single-dose vial, in a box of 10 single-dose vials, color coded with a green cap and stripe on the vial labels and cartons and an orange banner on the vial labels and cartons stating “Preservative Free”, NDC 0006-4995-41.
Syringes
No. 4094 — RECOMBIVAX HB for use in adults and adolescents (11 through 15 years of age) is supplied as 10 mcg/1.0 mL HBsAg in a carton of 6 single-dose prefilled Luer Lock syringes with tip caps, color coded with a green plunger rod and stripe on the peel-off syringe labels and cartons and an orange banner on the carton stating “Preservative Free”, NDC 0006-4094-09.
DIALYSIS FORMULATION (PRESERVATIVE FREE)
Vials
No. 4992 — RECOMBIVAX HB Dialysis Formulation is supplied as 40 mcg/mL of HBsAg in a 1 mL single-dose vial, color coded with a blue cap and stripe on the vial labels and cartons and an orange banner on the vial labels and cartons stating “Preservative Free”, NDC 0006-4992-00.
Storage
Store vials and syringes at 2-8°C (36-46°F). Storage above or below the recommended temperature may reduce potency.
Do not freeze since freezing destroys potency.
REFERENCES
- Robinson, W.S.: Hepatitis B Virus and the Delta Virus, in “Principles and Practice of Infectious Diseases,” G.L. Mandell; R.G. Douglas; J.E. Bennett (eds), vol. 2, New York, John Wiley & Sons, 1002-1029, 1985.
- Recommendation of the Immunization Practices Advisory Committee (ACIP): Protection Against Viral Hepatitis, MMWR 39(RR-2): 5-22, Feb. 9, 1990.
- Balistreri, W.F.: Viral Hepatitis, Unique Aspects of Infection During Childhood, Consultant 24(4): 131-153 passim, April 1984.
- Robinson, W.S.: Hepatitis B Virus and Hepatitis Delta Virus, in “Principles and Practice of Infectious Diseases,” G.L. Mandell, R.G. Douglas, and J.E. Bennett (eds), Churchill Livingstone, 1204-1231, 1990.
- Stevens, C.E.; Toy, P.T.; Tong, M.J.; Taylor, P.E.; Vyas, G.N.; Nair, P.V.; Gudavalli, M.; Krugman, S.: Perinatal Hepatitis B Virus Transmission in the United States, JAMA 253(12): 1740-1745, 1985.
- Beasley, R.P.; Hwang, L.; Stevens, C.E.; Lin, C.; Hsieh, F.; Wang, K.; Sun, T.; Szmuness, W.: Efficacy of Hepatitis B Immune Globulin for Prevention of Perinatal Transmission of the Hepatitis B Virus Carrier State: Final Report of a Randomized Double-Blind, Placebo-Controlled Trial, Hepatology 3: 135-141, 1983.
- Recommendations of the Immunization Practices Advisory Committee (ACIP): Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States Through Universal Childhood Vaccination, MMWR 40(RR-13): 1-25, November 22, 1991.
- Universal Hepatitis B Immunization, Committee on Infectious Diseases, Pediatrics 89(4): 795-800, 1992.
- Melnick, J.L.: Historical Aspects of Hepatitis B Vaccine, in “Hepatitis B Vaccine INSERM Symposium No. 18,” P. Maupas and P. Guesry (eds), Elsevier/North-Holland Biomedical Press, 23-31, 1981.
- Data on file at Merck Research Laboratories.
- Centers for Disease Control: Suboptimal Response to Hepatitis B Vaccine Given by Injection into the Buttock. MMWR 34(8): 105-113, March 1, 1985.
- Hadler, S.C., et al.: Long-term Immunogenicity and Efficacy of Hepatitis B Vaccine in Homosexual Men, NEJM 315: 209-214, 1986.
- Szmuness, W.; Stevens, C.E.; Horley, H.J., et al.: Hepatitis B Vaccine. Demonstration of Efficacy in a Controlled Clinical Trial in a High-risk Population in the United States. NEJM 303: 833-841, 1980.
- Francis, D.P.; Hadler, S.C.; Thompson, S.E., et al.: The Prevention of Hepatitis B with Vaccine. Report of the Centers for Disease Control Multi-center Efficacy Trial among Homosexual Men. Ann. Int. Med. 97: 362-366, 1982.
- Szmuness, W.; Stevens, C.E.; Horley, H.J., et al.: Hepatitis B Vaccine in Medical Staff of Hemodialysis Units. Efficacy and Subtype Cross-protection, NEJM 307: 1481-1486, 1982.
- Stevens, C.E.; Taylor, P.E.; Tong, M.J., et al.: Prevention of Perinatal Hepatitis B Virus Infection with Hepatitis B Immune Globulin and Hepatitis B Vaccine, in Zuckerman, A.J. (ed.), “Viral Hepatitis and Liver Diseases”, Alan R. Liss, 982-983, 1988.
- Stevens, C.E.; Taylor, P.E.; Tong, M.J., et al.: Yeast-Recombinant Hepatitis B Vaccine, Efficacy with Hepatitis B Immune Globulin in Prevention of Perinatal Hepatitis B Virus Transmission, JAMA 257(19): 2612-2616, 1987.
- Wainwright, R.B.; McMahon, B.J.; Bulkow, L.R., et al.: Duration of Immunogenicity and Efficacy of Hepatitis B Vaccine in a Yupik Eskimo Population, Preliminary Results of an 8-Year Study, in “Viral Hepatitis and Liver Disease,” F.B. Hollinger, S.M. Lemon, and H. Margolis (eds), Williams & Wilkins, 762-766, 1990.
- Hadler, S.C.; Coleman, P.J.; O'Malley, P., et al.: Evaluation of Long-Term Protection by Hepatitis B Vaccine for Seven to Nine Years in Homosexual Men, in “Viral Hepatitis and Liver Disease,” F.B. Hollinger, S.M. Lemon, and H. Margolis (eds), Williams & Wilkins, 766-768, 1990.
- Tong, M.J.; Stevens, C.E.; Taylor, P.E., et al.: Prevention of Hepatitis B Infection in Infants Born to HBeAg Positive HBsAg Carrier Mothers in the United States, in “An Update, 1989, Progress in Hepatitis B Immunization,” P. Coursaget and M.J. Tong (eds), Colloque INSERM/John Libbey Eurotext Ltd., Vol. 194, 339-345, 1990.
- Hwang, L-Y.; Lee, C-Y.; and Beasley, R.P.: Five-Year Follow-up of HBV Vaccination with Plasma-derived Vaccine in Neonates: Evaluation of Immunogenicity and Efficacy Against Perinatal Transmission, in “Viral Hepatitis and Liver Disease,” F.B. Hollinger, S.M. Lemon, and H. Margolis (eds), Williams & Wilkins, 759-761, 1990.
- West, D.J.; Calandra, G.B.: Vaccine Induced Immunologic Memory for Hepatitis B Surface Antigen; Implications for Policy on Booster Vaccination, Vaccine, 14(11): 1019-1027, 1996.
- Recommendations of the Immunization Practices Advisory Committee (ACIP): Update on Hepatitis B Prevention, MMWR 36(23): 353-366, June 19, 1987.
- Emini, E.A.; Ellis, R.W.; Miller, W.J.; McAleer, W.J.; Scolnick, E.M. and Gerety, R.J.: Production and Immunological Analysis of Recombinant Hepatitis B Vaccine, J. Infection, 13(Sup. A): 3-9, 1986.
- Brown, S.E.; Stanley, C.; Howard, C.R.; Zuckerman, A.J.; Steward, M.W.: Antibody Responses to Recombinant and Plasma- derived Hepatitis B Vaccines, Brit. Med. J., 292: 159-161, 1986.
- Yamamoto, S.; Kuroki, T.; Kurai, K.; Iino, S.: Comparison of Results for Phase I Studies with Recombinant and Plasma-derived Hepatitis B Vaccines, and Controlled Study Comparing Intramuscular and Subcutaneous Injections of Recombinant Hepatitis B Vaccine, J. Infection, 13(Sup. A): 53-60, 1986.
- Jilg, W.; Schmidt, M.; Zoulek, G.; Lorbeer, B.; Wilske, B.; Deinhardt, F.: Clinical Evaluation of a Recombinant Hepatitis B Vaccine, Lancet, 1174-1175, Nov. 24, 1984.
- Schalm, S.W.; Heytink, R.A.; Kruining, H.; Bakker-Bendik, M.: Immunogenicity of Recombinant Yeast Hepatitis-B Vaccine, Neth. J. Med. 29: 28, 1986.
- Centers for Disease Control: Epidemiology and Prevention of Vaccine-preventative Diseases, W. Atkinson, L. Furphy, J. Gantt, M. Mayfield, G. Phyne (eds), chapter 9.
- Recommendations of the Advisory Committee on Immunization Practices (ACIP): Hepatitis B Virus Infection: A Comprehensive Strategy to Eliminate Transmission in the United States, 1996 update, MMWR (draft January 13, 1996).
- Vaccine Adverse Event Reporting System - United States. MMWR 39(41): 730-733, October 19, 1990.
- Zajac, B.A.; West, D.J.; McAleer, W.J.; Scolnick, E.M.: Overview of Clinical Studies with Hepatitis B Vaccine Made by Recombinant DNA, J. Infection, 13(Sup. A): 39-45, July 1986.
- WHO Bulletin, Expanded Programme on Immunization, Hepatitis B Vaccine – Making Global Progress. October, 1996.
- Centers for Disease Control and Prevention, Federal Register, February 23, 1999, 64(35): 9044-9045.
- National Institutes of Health, National Institutes of Health Consensus Development Conference Panel Statement: Management of Hepatitis C, Hepatology, 26(Suppl. 1): 2S-10S, 1997.
- Wiedmann, M.; Liebert, U.G.; Oesen, U.; Porst, H.; Wiese, M.; Schroeder, S.; Halm, U.; Mossner, J.; Berr, F.: Decreased Immunogenicity of Recombinant Hepatitis B Vaccine in Chronic Hepatitis C, Hepatology, 31: 230-234, 2000.
- Minniti, F.; Baldo, V.; Trivello, R.; Bricolo, R.; Di Furia, L.; Renzulli, G.; Chiaramonte, M.: Response to HBV vaccine in Relation to anti-HCV and anti-HBc Positivity: a Study in Intravenous Drug Addicts, Vaccine, 17: 3083-3085, 1999.
Manuf. and Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Issued December 2010
Printed in USA
9987434
Copyright © 1998 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved
This is a representative sample of the packaging. Please see How Supplied section for a complete list of available packaging.
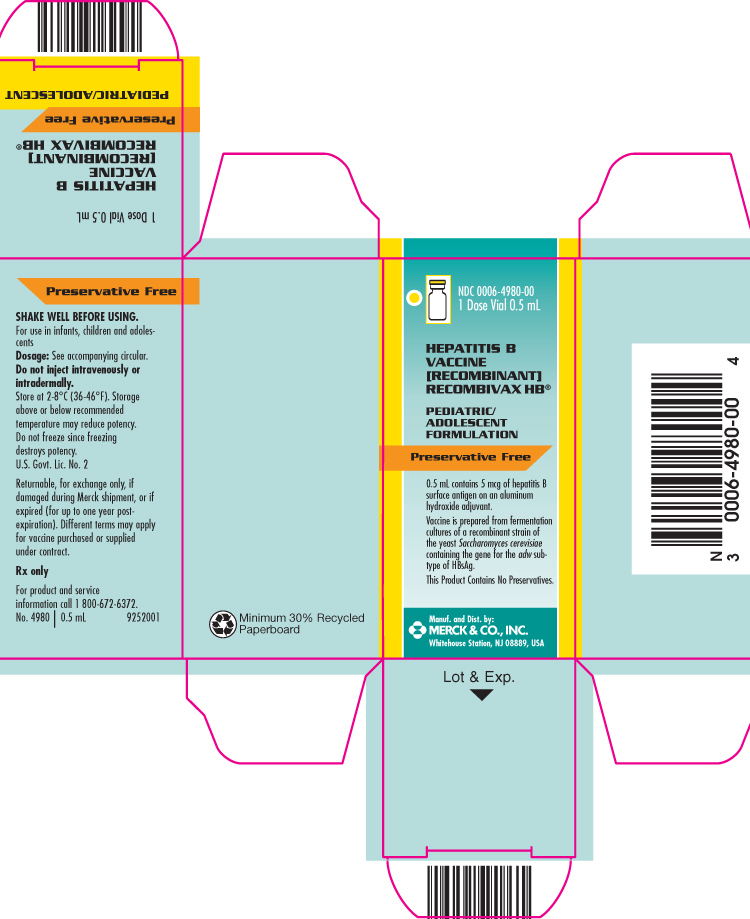
PRINCIPAL DISPLAY PANEL - Carton - 1 Dose Vial 0.5 mL
NDC 0006-4980-00
1 Dose Vial 0.5 mL
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
PEDIATRIC/ADOLESCENT FORMULATION
Preservative Free
0.5 mL contains 5 mcg of hepatitis B surface antigen on an aluminum hydroxide adjuvant.
Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg.
This Product Contains No Preservatives.
Manuf. and Dist. by:
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA
PRINCIPAL DISPLAY PANEL - Carton - 10 Single-Dose 0.5 mL Vials
NDC 0006-4981-00
10 Single-Dose 0.5 mL Vials
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
PEDIATRIC/ADOLESCENT
Preservative Free
This package contains 10 single-dose vials. Each 0.5 mL vial contains 5 mcg of hepatitis B surface antigen on an aluminum hydroxide adjuvant. Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg. This Product Contains No Preservatives.
Manuf. and Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA

PRINCIPAL DISPLAY PANEL - Carton - 6 Single-Dose 0.5 mL Syringes
NDC 0006-4093-09
6 Single-Dose 0.5 mL Syringes
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
PEDIATRIC/ADOLESCENT FORMULATION
Preservative Free
This package contains 6 single-dose syringes. Each 0.5 mL syringe contains 5 mcg of hepatitis B surface antigen adjuvanted with amorphous aluminum hydroxyphosphate sulfate.
Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg.
This Product Contains No Thimerosal.
Rx only
Manuf. and Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA

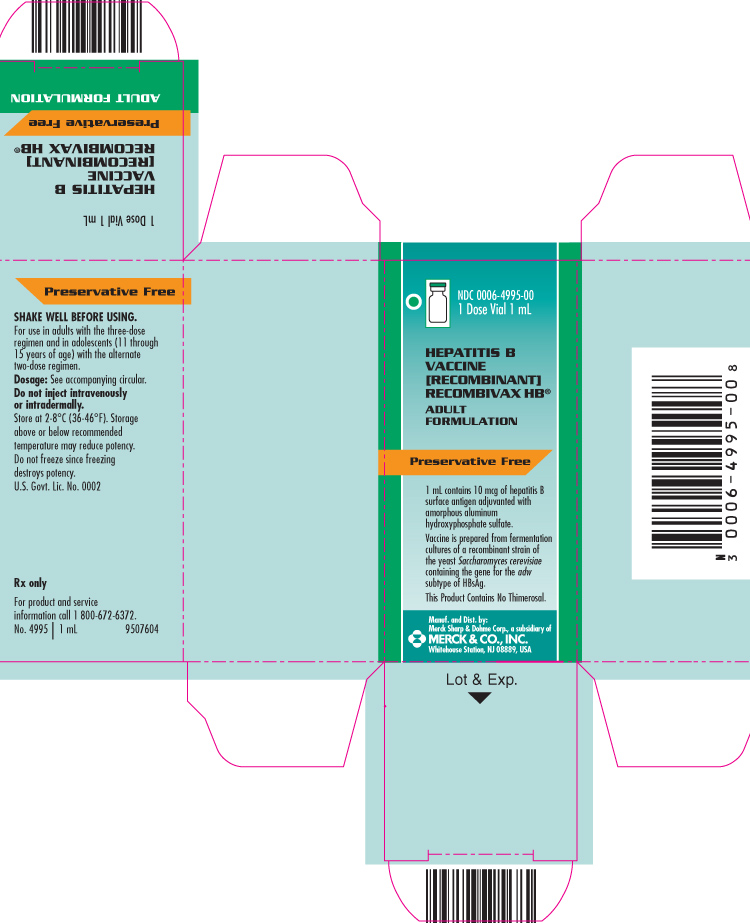
PRINCIPAL DISPLAY PANEL - Carton - 1 Dose Vial 1 mL
NDC 0006-4995-00
1 Dose Vial 1 mL
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
ADULT FORMULATION
Preservative Free
1 mL contains 10 mcg of hepatitis B surface antigen adjuvanted with amorphous aluminum hydroxyphosphate sulfate.
Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg.
This Product Contains No Thimerosal.
Manuf. and Dist. by:
Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA
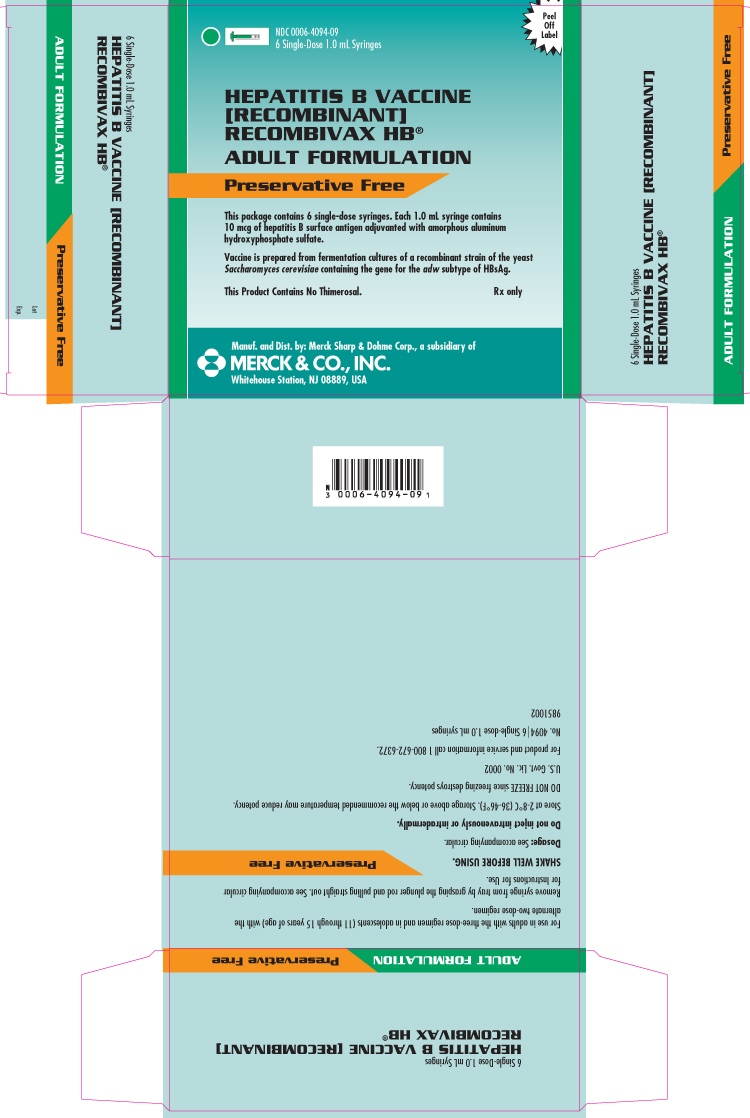
PRINCIPAL DISPLAY PANEL - Carton - 6 Single-Dose 1.0 mL Syringes
NDC 0006-4094-09
6 Single-Dose 1.0 mL Syringes
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
ADULT FORMULATION
Preservative Free
This package contains 6 single-dose syringes. Each 1.0 mL syringe contains 10 mcg of hepatitis B surface antigen adjuvanted with amorphous aluminum hydroxyphosphate sulfate.
Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg.
This Product Contains No Thimerosal.
Rx only
Manuf. and Dist. by: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA
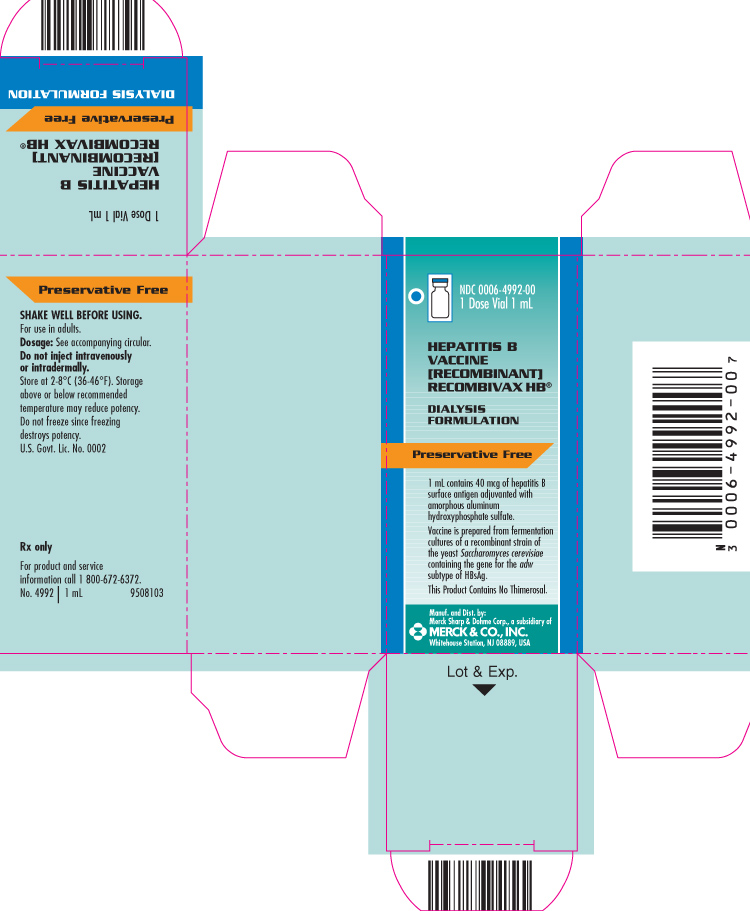
PRINCIPAL DISPLAY PANEL - Carton - 1 Dose Vial 1 mL
NDC 0006-4992-00
1 Dose Vial 1 mL
HEPATITIS B VACCINE [RECOMBINANT]
RECOMBIVAX HB®
DIALYSIS FORMULATION
Preservative Free
1 mL contains 40 mcg of hepatitis B surface antigen adjuvanted with amorphous aluminum hydroxyphosphate sulfate.
Vaccine is prepared from fermentation cultures of a recombinant strain of the yeast Saccharomyces cerevisiae containing the gene for the adw subtype of HBsAg.
This Product Contains No Thimerosal.
Manuf. and Dist. by:
Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| RECOMBIVAX HB
hepatitis b vaccine (recombinant) injection, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA101066 | 07/23/1986 | |
| Labeler - Merck Sharp & Dohme Corp. (001317064) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Merck Sharp & Dohme Corp. | 002387926 | MANUFACTURE | |