sensipar (cinacalcet hydrochloride) tablet, coated
[Amgen Inc.]
DESCRIPTION
Sensipar® (cinacalcet hydrochloride) is a calcimimetic agent that increases the sensitivity of the calcium-sensing receptor to activation by extracellular calcium. Its empirical formula is C22H22F3N•HCl with a molecular weight of 393.9 g/mol (hydrochloride salt) and 357.4 g/mol (free base). It has one chiral center having an R-absolute configuration. The Renantiomer is the more potent enantiomer and has been shown to be responsible for pharmacodynamic activity.
Cinacalcet HCl is a white to off-white, crystalline solid that is soluble in methanol or 95% ethanol and slightly soluble in water.
Sensipar® tablets are formulated as light-green, film-coated, oval-shaped tablets for oral administration in strengths of 30 mg, 60 mg, and 90 mg of cinacalcet HCl as the free base equivalent (33 mg, 66 mg, and 99 mg as the hydrochloride salt, respectively).
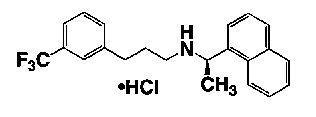
Cinacalcet HCl is described chemically as N-[1-(R)-(-)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-aminopropane hydrochloride and has the following structural formula:
Inactive Ingredients: Sensipar® tablets are comprised of the active ingredient, and the following inactive ingredients: pre-gelatinized starch, microcrystalline cellulose, povidone, crospovidone, colloidal silicon dioxide, and magnesium stearate. Tablets are coated with color (Opadry®II green) and clear film-coat (Opadry® clear), carnauba wax, and Opacode® black ink.
CLINICAL PHARMACOLOGY
Mechanism of Action
Secondary hyperparathyroidism (HPT) in patients with chronic kidney disease (CKD) is a progressive disease, associated with increases in parathyroid hormone (PTH) levels and derangements in calcium and phosphorus metabolism. Increased PTH stimulates osteoclastic activity resulting in cortical bone resorption and marrow fibrosis. The goals of treatment of secondary hyperparathyroidism are to lower levels of PTH, calcium, and phosphorus in the blood, in order to prevent progressive bone disease and the systemic consequences of disordered mineral metabolism. In CKD patients on dialysis with uncontrolled secondary HPT, reductions in PTH are associated with a favorable impact on bone-specific alkaline phosphatase (BALP), bone turnover and bone fibrosis.
The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH secretion. Sensipar® directly lowers PTH levels by increasing the sensitivity of the calcium-sensing receptor to extracellular calcium. The reduction in PTH is associated with a concomitant decrease in serum calcium levels.
Pharmacokinetics
Absorption and Distribution:
After oral administration of cinacalcet, maximum plasma concentration (Cmax) is achieved in approximately 2 to 6 hours. A food-effect study in healthy volunteers indicated that the Cmax and area under the curve (AUC(0-inf)) were increased 82% and 68%, respectively, when cinacalcet was administered with a high-fat meal compared to fasting. Cmax and AUC(0-inf) of cinacalcet were increased 65% and 50%, respectively, when cinacalcet was administered with a low-fat meal compared to fasting.
After absorption, cinacalcet concentrations decline in a biphasic fashion with a terminal half-life of 30 to 40 hours. Steady-state drug levels are achieved within 7 days. The mean accumulation ratio is approximately 2 with once-daily oral administration. The median accumulation ratio is approximately 2 to 5 with twice-daily oral administration. The AUC and Cmax of cinacalcet increase proportionally over the dose range of 30 to 180 mg once daily. The pharmacokinetic profile of cinacalcet does not change over time with once-daily dosing of 30 to 180 mg. The volume of distribution is high (approximately 1000 L), indicating extensive distribution. Cinacalcet is approximately 93 to 97% bound to plasma protein(s). The ratio of blood cinacalcet concentration to plasma cinacalcet concentration is 0.80 at a blood cinacalcet concentration of 10 ng/mL.
Metabolism and Excretion:
Cinacalcet is metabolized by multiple enzymes, primarily CYP3A4, CYP2D6 and CYP1A2. After administration of a 75 mg radiolabeled dose to healthy volunteers, cinacalcet was rapidly and extensively metabolized via: 1) oxidative N-dealkylation to hydrocinnamic acid and hydroxy-hydrocinnamic acid, which are further metabolized via β-oxidation and glycine conjugation; the oxidative N-dealkylation process also generates metabolites that contain the naphthalene ring; and 2) oxidation of the naphthalene ring on the parent drug to form dihydrodiols, which are further conjugated with glucuronic acid. The plasma concentrations of the major circulating metabolites including the cinnamic acid derivatives and glucuronidated dihydrodiols markedly exceed parent drug concentrations. The hydrocinnamic acid metabolite was shown to be inactive at concentrations up to 10 µM in a cell-based assay measuring calcium-receptor activation. The glucuronide conjugates formed after cinacalcet oxidation were shown to have a potency approximately 0.003 times that of cinacalcet in a cell-based assay measuring a calcimimetic response. Renal excretion of metabolites was the primary route of elimination of radioactivity. Approximately 80% of the dose was recovered in the urine and 15% in the feces.
Special Populations
Hepatic Insufficiency:
The disposition of a 50 mg cinacalcet single dose was compared in patients with hepatic impairment and subjects with normal hepatic function. Cinacalcet exposure, AUC(0-inf), was comparable between healthy volunteers and patients with mild hepatic impairment. However, in patients with moderate and severe hepatic impairment (as indicated by the Child-Pugh method), cinacalcet exposures as defined by the AUC(0-inf) were 2.4 and 4.2 times higher, respectively, than that in normals. The mean half-life of cinacalcet is prolonged by 33% and 70% in patients with moderate and severe hepatic impairment, respectively. Protein binding of cinacalcet is not affected by impaired hepatic function. See PRECAUTIONS and DOSAGE AND ADMINISTRATION.
Renal Insufficiency:
The pharmacokinetic profile of a 75 mg Sensipar® single dose in patients with mild, moderate, and severe renal insufficiency, and those on hemodialysis or peritoneal dialysis is comparable to that in healthy volunteers.
Geriatric Patients:
The pharmacokinetic profile of Sensipar® in geriatric patients (age ≥ 65, n = 12) is similar to that for patients who are < 65 years of age (n = 268).
Pediatric Patients:
The pharmacokinetics of Sensipar® have not been studied in patients < 18 years of age.
Drug Interactions
An in vitro study indicates that cinacalcet is a strong inhibitor of CYP2D6, but not of CYP1A2, CYP2C9, CYP2C19, and CYP3A4.
Ketoconazole: Cinacalcet AUC(0-inf) and Cmax increased 2.3 and 2.2 times, respectively, when a single 90 mg cinacalcet dose on Day 5 was administered to subjects treated with 200 mg ketoconazole twice daily for 7 days compared to 90 mg cinacalcet given alone (see DOSAGE AND ADMINISTRATION).
Calcium Carbonate: No significant pharmacokinetic interaction was observed when 1500 mg calcium carbonate was coadministered with 100 mg cinacalcet.
Pantoprazole: No significant pharmacokinetic interaction was observed when cinacalcet 90 mg was administered to subjects treated with 80 mg pantoprazole daily for 3 days.
Sevelamer HCl: No significant pharmacokinetic interaction was observed when 2400 mg sevelamer HCl was coadministered with 90 mg cinacalcet tablet (subjects subsequently received 2400 mg sevelamer HCl two more times on Day 1 and three more times on Day 2).
Amitriptyline: Concurrent administration of 25 mg or 100 mg cinacalcet with 50 mg amitriptyline increased amitriptyline exposure and nortriptyline (active metabolite) exposure by approximately 20% in CYP2D6 extensive metabolizers.
Warfarin: R- and S-warfarin pharmacokinetics and warfarin pharmacodynamics were not affected in subjects treated with warfarin 25 mg who received cinacalcet 30 mg twice daily. The lack of effect of cinacalcet on the pharmacokinetics of R- and S-warfarin and the absence of auto-induction upon multiple dosing in patients indicates that cinacalcet is not an inducer of CYP2C9 in humans.
Pharmacodynamics
Reduction in intact PTH (iPTH) levels correlated with cinacalcet concentrations in CKD patients. The nadir in iPTH level occurs approximately 2 to 6 hours post dose, corresponding with the Cmax of cinacalcet. After steady state is reached, serum calcium concentrations remain constant over the dosing interval in CKD patients.
CLINICAL STUDIES
Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
Three 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies of similar design were conducted in CKD patients on dialysis. A total of 665 patients were randomized to Sensipar® and 471 patients to placebo. The mean age of the patients was 54 years, 62% were male, and 52% Caucasian. The average baseline iPTH level by the Nichols intact immunoradiometric assay (IRMA) was 712 pg/mL, with 26% of the patients having a baseline iPTH level > 800 pg/mL. The mean baseline Ca x P ion product was 61 mg2/dL2. The average duration of dialysis prior to study enrollment was 67 months. Ninety-six percent of patients were on hemodialysis and 4% peritoneal dialysis. At study entry, 66% of the patients were receiving vitamin D sterols and 93% were receiving phosphate binders. Sensipar® (or placebo) was initiated at a dose of 30 mg once daily and titrated every 3 or 4 weeks to a maximum dose of 180 mg once daily to achieve an iPTH of ≤ 250 pg/mL. The dose was not increased if a patient had any of the following: iPTH < 200 pg/mL, serum calcium < 7.8 mg/dL, or any symptoms of hypocalcemia. If a patient experienced symptoms of hypocalcemia or had a serum calcium < 8.4 mg/dL, calcium supplements and/or calcium-based phosphate binders could be increased. If these measures were insufficient, the vitamin D dose could be increased. Approximately 70% of the Sensipar® patients and 80% of the placebo patients completed the 6-month studies. In the primary efficacy analysis, 40% of Sensipar® patients and 5% of placebo patients achieved an iPTH ≤ 250 pg/mL (p<0.001) (Table 1, Figure 1). Secondary efficacy parameters also improved in patients treated with Sensipar®. These studies showed that Sensipar® reduced PTH while lowering Ca x P, calcium and phosphorus levels (Table 1, Figure 2). The median dose of Sensipar® at the completion of the studies was 90 mg. Patients with milder disease typically required lower doses.
| Study 1 | Study 2 | Study 3 | ||||||
| Placebo | Sensipar® | Placebo | Sensipar® | Placebo | Sensipar® | |||
| (N = 205) | (N = 205) | (N = 165) | (N = 166) | (N = 101) | (N = 294) | |||
| iPTH | ||||||||
|
Baseline (pg/mL): Median Mean (SD) |
535 651 (398) |
537 636 (341) |
556 630 (317) |
547 652 (372) |
670 832 (486) |
703 848 (685) |
||
| Evaluation Phase (pg/mL) | 563 | 275 | 592 | 238 | 737 | 339 | ||
| Median Percent Change | +3.8 | -48.3 | +8.4 | -54.1 | +2.3 | -48.2 | ||
| Patients Achieving Primary Endpoint (iPTH ≤ 250 pg/mL) (%)* | 4% | 41%† | 7% | 46%† | 6% | 35%† | ||
| Patients Achieving ≥ 30% Reduction in iPTH (%)* | 11% | 61% | 12% | 68% | 10% | 59% | ||
| Patients Achieving iPTH ≤ 250 pg/mL and Ca x P < 55 mg2/dL2 (%) | 1% | 32% | 5% | 35% | 5% | 28% | ||
| Ca x P | ||||||||
| Baseline (mg2/dL2) | 62 | 61 | 61 | 61 | 61 | 59 | ||
| Evaluation Phase (mg2/dL2) | 59 | 52 | 59 | 47 | 57 | 48 | ||
| Median Percent Change | -2.0 | -14.9 | -3.1 | -19.7 | -4.8 | -15.7 | ||
| Calcium | ||||||||
| Baseline (mg/dL) 9.8 | 9.8 | 9.8 | 9.9 | 10.0 | 9.9 | 9.8 | ||
| Evaluation Phase (mg/dL) 9.9 | 9.9 | 9.1 | 9.9 | 9.1 | 10.0 | 9.1 | ||
| Median Percent Change +0.5 | +0.5 | -5.5 | +0.1 | -7.4 | +0.3 | -6.0 | ||
| Phosphorus | ||||||||
| Baseline (mg/dL) 6.3 | 6.3 | 6.1 | 6.1 | 6.0 | 6.1 | 6.0 | ||
| Evaluation Phase (mg/dL) 6.0 | 6.0 | 5.6 | 5.9 | 5.1 | 5.6 | 5.3 | ||
| Median Percent Change -1.0 | -1.0 | -9.0 | -2.4 | -12.4 | -5.6 | -8.6 | ||
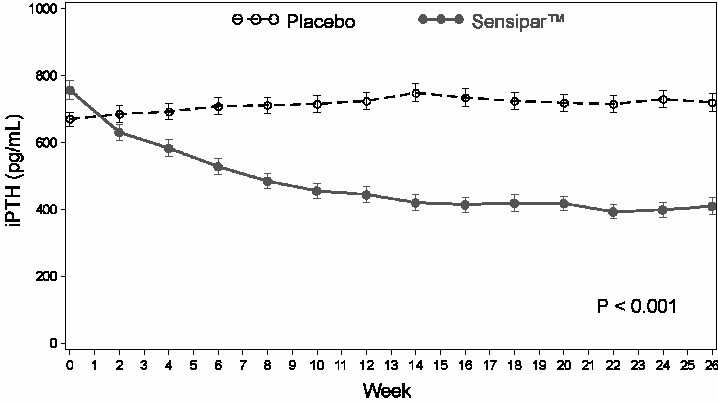
Figure 1. Mean (SE) iPTH Values (Pooled Phase 3 Studies) Data are presented for patients who completed the studies; Placebo (N = 342), Sensipar® (N = 439).
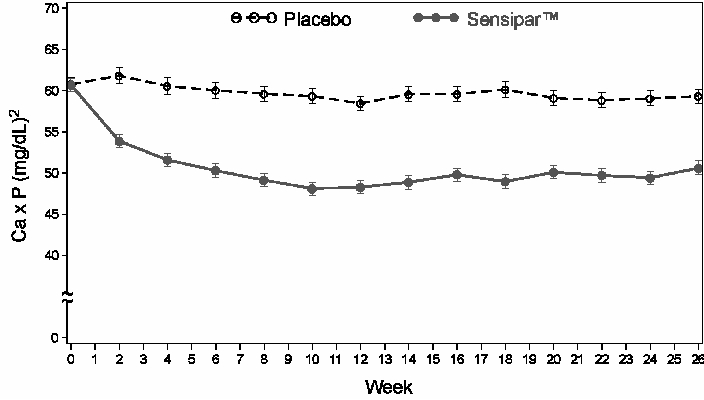
Figure 2. Mean (SE) Ca x P Values (Pooled Phase 3 Studies) Data are presented for patients who completed the studies; Placebo (N = 342), Sensipar® (N = 439).
Reductions in iPTH and Ca x P were maintained for up to 12 months of treatment.
Sensipar® decreased iPTH and Ca x P levels regardless of disease severity (i.e., baseline iPTH value), duration of dialysis, and whether or not vitamin D sterols were administered. Approximately 60% of patients with mild (iPTH ≥ 300 to ≤ 500 pg/mL), 41% with moderate (iPTH > 500 to 800 pg/mL), and 11% with severe (iPTH > 800 pg/mL) secondary HPT achieved a mean iPTH value of 250 pg/mL. Plasma iPTH levels were measured using the Nichols IRMA.
Parathyroid Carcinoma
Ten patients with parathyroid carcinoma were enrolled in an open-label study. The study consisted of 2 phases, a dose-titration phase and a maintenance phase.
The range of exposure was 2 to 16 weeks in the titration phase (n = 10) and 16 to 48 weeks (n = 3) for the maintenance phase. Baseline mean (SD) serum calcium was 14.7 (1.8) mg/dL. The range of change from baseline to last measurement was –7.5 to 2.7 mg/dL during the titration phase and –7.4 to 0.9 mg/dL during the maintenance phase ( Figure 3). No patients maintained a serum calcium level within the normal range. The doses ranged from 70 mg twice daily to 90 mg four times daily for patients in the maintenance phase.
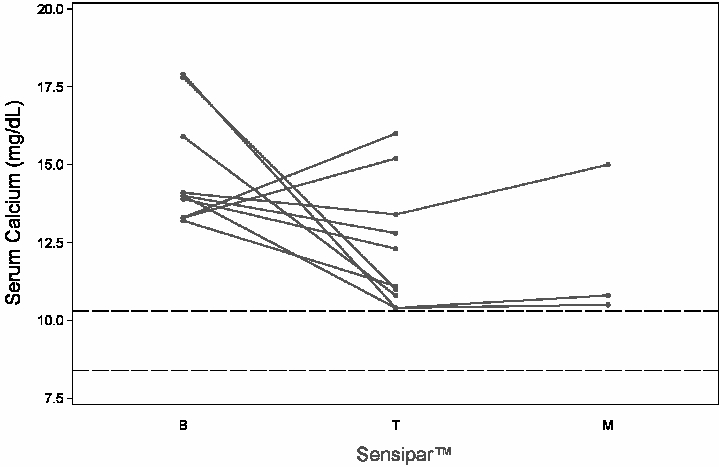
Figure 3. Serum Calcium Values in Parathyroid Carcinoma Patients Receiving Sensipar® at Baseline, Titration and Maintenance Phase Solid lines represent individual patient data B = baseline; T = last value in titration phase; M = last value in maintenance phase Reference lines (dashed) show the normal range for serum calcium values
INDICATIONS AND USAGE
Sensipar® is indicated for the treatment of secondary hyperparathyroidism in patients with Chronic Kidney Disease on dialysis.
Sensipar® is indicated for the treatment of hypercalcemia in patients with parathyroid carcinoma.
CONTRAINDICATIONS
Sensipar® is contraindicated in patients with hypersensitivity to any component(s) of this product.
WARNINGS
Seizures
In three clinical studies of CKD patients on dialysis, 5% of the patients in both the Sensipar® and placebo groups reported a history of seizure disorder at baseline. During the trials, seizures (primarily generalized or tonic-clonic) were observed in 1.4% (9/656) of Sensipar®-treated patients and 0.4% (2/470) of placebo-treated patients. Five of the nine Sensipar®-treated patients had a history of a seizure disorder and two were receiving anti-seizure medication at the time of their seizure. Both placebo-treated patients had a history of seizure disorder and were receiving anti-seizure medication at the time of their seizure. While the basis for the reported difference in seizure rate is not clear, the threshold for seizures is lowered by significant reductions in serum calcium levels. Therefore, serum calcium levels should be closely monitored in patients receiving Sensipar®, particularly in patients with a history of a seizure disorder (see PRECAUTIONS, Hypocalcemia).
Hypotension and/or Worsening Heart Failure
In postmarketing safety surveillance, isolated, idiosyncratic cases of hypotension and/or worsening heart failure have been reported in patients with impaired cardiac function, in which a causal relationship to Sensipar® could not be completely excluded and may be mediated by reductions in serum calcium levels. Clinical trial data showed hypotension occurred in 7% of Sensipar®-treated patients, 12% of placebo-treated patients, and heart failure occurred in 2% of patients receiving Sensipar® or placebo.
PRECAUTIONS
General
Hypocalcemia
Sensipar® lowers serum calcium, and therefore patients should be carefully monitored for the occurrence of hypocalcemia. Potential manifestations of hypocalcemia include paresthesias, myalgias, cramping, tetany, and convulsions.
Sensipar® treatment should not be initiated if serum calcium is less than the lower limit of the normal range (8.4 mg/dL). Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar®. Once the maintenance dose has been established, serum calcium should be measured approximately monthly (see DOSAGE AND ADMINISTRATION).
If serum calcium falls below 8.4 mg/dL but remains above 7.5 mg/dL, or if symptoms of hypocalcemia occur, calcium-containing phosphate binders and/or vitamin D sterols can be used to raise serum calcium. If serum calcium falls below 7.5 mg/dL, or if symptoms of hypocalcemia persist and the dose of vitamin D cannot be increased, withhold administration of Sensipar® until serum calcium levels reach 8.0 mg/dL, and/or symptoms of hypocalcemia have resolved. Treatment should be re-initiated using the next lowest dose of Sensipar® (see DOSAGE AND ADMINISTRATION).
In the 26-week studies of patients with CKD on dialysis, 66% of patients receiving Sensipar® compared with 25% of patients receiving placebo developed at least one serum calcium value < 8.4 mg/dL. Less than 1% of patients in each group permanently discontinued study drug due to hypocalcemia.
Sensipar® is not indicated for CKD patients not on dialysis. In CKD patients with secondary HPT not on dialysis, the long-term safety and efficacy of Sensipar® have not been established. Clinical studies indicate that Sensipar®-treated CKD patients not on dialysis have an increased risk for hypocalcemia compared to Sensipar®-treated CKD patients on dialysis, which may be due to lower baseline calcium levels. In a phase 3 study of 32 weeks duration and including 404 subjects (302 cinacalcet, 102 placebo), in which the median dose for cinacalcet was 60 mg at the completion of the study, 80% of Sensipar®-treated patients experienced at least one serum calcium value < 8.4 mg/dL compared to 5% of patients receiving placebo.
Adynamic Bone Disease
Adynamic bone disease may develop if iPTH levels are suppressed below 100 pg/mL when assessed using the standard Nichols IRMA. One clinical study evaluated bone histomorphometry in patients treated with Sensipar® for one year. Three patients with mild hyperparathyroid bone disease at the beginning of the study developed adynamic bone disease during treatment with Sensipar®. Two of these patients had iPTH levels below 100 pg/mL at multiple time points during the study. In the three 6-month, phase 3 studies conducted in CKD patients on dialysis, 11% of patients treated with Sensipar® had mean iPTH values below 100 pg/mL during the efficacy-assessment phase. If iPTH levels decrease below the NKF-K/DOQI recommended target range (150-300 pg/mL) 1 in patients treated with Sensipar®, the dose of Sensipar® and/or vitamin D sterols should be reduced or therapy discontinued.
Hepatic Insufficiency
Cinacalcet exposure as assessed by AUC(0-inf) in patients with moderate and severe hepatic impairment (as indicated by the Child-Pugh method) were 2.4 and 4.2 times higher, respectively, than that in normals. Patients with moderate and severe hepatic impairment should be monitored throughout treatment with Sensipar® (see CLINICAL PHARMACOLOGY, Pharmacokinetics and DOSAGE AND ADMINISTRATION).
Information for Patients
It is recommended that Sensipar® be taken with food or shortly after a meal. Tablets should be taken whole and should not be divided.
Laboratory tests
Patients with CKD on Dialysis with Secondary Hyperparathyroidism
Serum calcium and serum phosphorus should be measured within 1 week and iPTH should be measured 1 to 4 weeks after initiation or dose adjustment of Sensipar®. Once the maintenance dose has been established, serum calcium and serum phosphorus should be measured approximately monthly, and PTH every 1 to 3 months (see DOSAGE AND ADMINISTRATION). All iPTH measurements during the Sensipar® trials were obtained using the Nichols IRMA.
In patients with end-stage renal disease, testosterone levels are often below the normal range. In a placebo-controlled trial in patients with CKD on dialysis, there were reductions in total and free testosterone in male patients following six months of treatment with Sensipar®. Levels of total testosterone decreased by a median of 15.8% in the Sensipar®-treated patients and by 0.6% in the placebo-treated patients. Levels of free testosterone decreased by a median of 31.3% in the Sensipar®-treated patients and by 16.3% in the placebo-treated patients. The clinical significance of these reductions in serum testosterone is unknown.
Patients with Parathyroid Carcinoma
Serum calcium should be measured within 1 week after initiation or dose adjustment of Sensipar®. Once maintenance dose levels have been established, serum calcium should be measured every 2 months (see DOSAGE AND ADMINISTRATION).
Drug Interactions and/or Drug/Laboratory Test Interactions
See CLINICAL PHARMACOLOGY, Pharmacokinetics and Drug Interactions.
Effect of Sensipar® on other drugs:
Drugs metabolized by cytochrome P450 2D6 (CYP2D6): Sensipar® is a strong in vitro inhibitor of CYP2D6. Therefore, dose adjustments of concomitant medications that are predominantly metabolized by CYP2D6 and have a narrow therapeutic index (e.g., flecainide, vinblastine, thioridazine and most tricyclic antidepressants) may be required.
Amitriptyline: Concurrent administration of 25 mg or 100 mg cinacalcet with 50 mg amitriptyline increased amitriptyline exposure and nortriptyline (active metabolite) exposure by approximately 20% in CYP2D6 extensive metabolizers.
Effect of other drugs on Sensipar®:
Sensipar® is metabolized by multiple cytochrome P450 enzymes, primarily CYP3A4, CYP2D6, and CYP1A2.
Ketoconazole: Sensipar® is metabolized in part by CYP3A4. Co-administration of ketoconazole, a strong inhibitor of CYP3A4, increased cinacalcet exposure following a single 90 mg dose of Sensipar® by 2.3 fold. Dose adjustment of Sensipar® may be required and PTH and serum calcium concentrations should be closely monitored if a patient initiates or discontinues therapy with a strong CYP3A4 inhibitor (e.g., ketoconazole, erythromycin, itraconazole; see DOSAGE AND ADMINISTRATION).
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenicity:
Standard lifetime dietary carcinogenicity bioassays were conducted in mice and rats. Mice were given dietary doses of 15, 50, 125 mg/kg/day in males and 30, 70, 200 mg/kg/day in females (exposures up to 2 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). Rats were given dietary doses of 5, 15, 35 mg/kg/day in males and 5, 20, 35 mg/kg/day in females (exposures up to 2 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). No increased incidence of tumors was observed following treatment with cinacalcet.
Mutagenicity:
Cinacalcet was not genotoxic in the Ames bacterial mutagenicity assay or in the Chinese Hamster Ovary (CHO) cell HGPRT forward mutation assay and CHO cell chromosomal aberration assay, with and without metabolic activation or in the in vivo mouse micronucleus assay.
Impairment of Fertility:
Female rats were given oral gavage doses of 5, 25, 75 mg/kg/day beginning 2 weeks before mating and continuing through gestation day 7. Male rats were given oral doses 4 weeks prior to mating, during mating (3 weeks) and 2 weeks post-mating. No effects were observed in male or female fertility at 5 and 25 mg/kg/day (exposures up to 3 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). At 75 mg/kg/day, there were slight adverse effects (slight decreases in body weight and food consumption) in males and females.
Pregnancy Category C
In pregnant female rats given oral gavage doses of 2, 25, 50 mg/kg/day during gestation no teratogenicity was observed at doses up to 50 mg/kg/day (exposure 4 times those resulting with a human oral dose of 180 mg/day based on AUC comparison). Decreased fetal body weights were observed at all doses (less than 1 to 4 times a human oral dose of 180 mg/day based on AUC comparison) in conjunction with maternal toxicity (decreased food consumption and body weight gain).
In pregnant female rabbits given oral gavage doses of 2, 12, 25 mg/kg/day during gestation no adverse fetal effects were observed (exposures less than with a human oral dose of 180 mg/day based on AUC comparisons). Reductions in maternal food consumption and body weight gain were seen at doses of 12 and 25 mg/kg/day.
In pregnant rats given oral gavage doses of 5, 15, 25 mg/kg/day during gestation through lactation no adverse fetal or pup (post-weaning) effects were observed at 5 mg/kg/day (exposures less than with a human therapeutic dose of 180 mg/day based on AUC comparisons). Higher doses of 15 and 25 mg/kg/day (exposures 2-3 times a human oral dose of 180 mg/day based on AUC comparisons) were accompanied by maternal signs of hypocalcemia (periparturient mortality and early postnatal pup loss), and reductions in postnatal maternal and pup body-weight gain. Sensipar® has been shown to cross the placental barrier in rabbits.
There are no adequate and well-controlled studies in pregnant women. Sensipar® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactating Women
Studies in rats have shown that Sensipar® is excreted in the milk with a high milk-to-plasma ratio. It is not known whether this drug is excreted in human milk. Considering these data in rats and because many drugs are excreted in human milk and because of the potential for clinically significant adverse reactions in infants from Sensipar®, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the lactating woman.
Pediatric Use
The safety and efficacy of Sensipar® in pediatric patients have not been established.
Geriatric Use
Of the 1136 patients enrolled in the Sensipar® phase 3 clinical program, 26% were ≥ 65 years old, and 9% were ≥ 75 years old. No differences in the safety and efficacy of Sensipar® were observed in patients greater or less than 65 years of age (see DOSAGE AND ADMINISTRATION, Geriatric Patients).
ADVERSE EVENTS
Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
In 3 double-blind placebo-controlled clinical trials, 1126 CKD patients on dialysis received study drug (656 Sensipar®, 470 placebo) for up to 6 months. The most frequently reported adverse events (incidence of at least 5% in the Sensipar® group and greater than placebo) are provided in Table 2. The most frequently reported events in the Sensipar® group were nausea, vomiting, and diarrhea.
| Placebo | Sensipar® | ||
| (n = 470) | (n = 656) | ||
| Event*: | (%) | (%) | |
|
|||
| Nausea | 19 | 31 | |
| Vomiting | 15 | 27 | |
| Diarrhea | 20 | 21 | |
| Myalgia | 14 | 15 | |
| Dizziness | 8 | 10 | |
| Hypertension | 5 | 7 | |
| Asthenia | 4 | 7 | |
| Anorexia | 4 | 6 | |
| Pain Chest, NonCardiac | 4 | 6 | |
| Access Infection | 4 | 5 | |
The incidence of serious adverse events (29% vs. 31%) was similar in the Sensipar® and placebo groups, respectively.
12-Month Experience with Sensipar®: Two hundred and sixty-six patients from 2 phase 3 studies continued to receive Sensipar® or placebo treatment in a 6-month double-blind extension study (12-month total treatment duration). The incidence and nature of adverse events in this study were similar in the two treatment groups, and comparable to those observed in the phase 3 studies.
Postmarketing Experience with Sensipar®: Isolated, idiosyncratic cases of hypotension and/or worsening heart failure have been reported in Sensipar®-treated patients with impaired cardiac function in postmarketing safety surveillance. Diarrhea and myalgia have been identified as adverse reactions during post-approval use of Sensipar®. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Parathyroid Carcinoma
The most frequent adverse events in this patient group were nausea and vomiting.
Laboratory values: Serum calcium levels should be closely monitored in patients receiving Sensipar® (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
OVERDOSAGE
Doses titrated up to 300 mg once daily have been safely administered to patients on dialysis. Overdosage of Sensipar® may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to correct serum calcium levels (see PRECAUTIONS).
Since Sensipar® is highly protein bound, hemodialysis is not an effective treatment for overdosage of Sensipar®.
DOSAGE AND ADMINISTRATION
Sensipar® tablets should be taken whole and should not be divided. Sensipar® should be taken with food or shortly after a meal.
Dosage must be individualized.
Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis
The recommended starting oral dose of Sensipar® is 30 mg once daily. Serum calcium and serum phosphorus should be measured within 1 week and iPTH should be measured 1 to 4 weeks after initiation or dose adjustment of Sensipar®. Sensipar® should be titrated no more frequently than every 2 to 4 weeks through sequential doses of 60, 90, 120, and 180 mg once daily to target iPTH consistent with the NKF-K/DOQI recommendation for CKD patients on dialysis of 150-300 pg/mL.
Sensipar® can be used alone or in combination with vitamin D sterols and/or phosphate binders.
During dose titration, serum calcium levels should be monitored frequently and if levels decrease below the normal range, appropriate steps should be taken to increase serum calcium levels, such as by providing supplemental calcium, initiating or increasing the dose of calcium-based phosphate binder, initiating or increasing the dose of vitamin D sterols, or temporarily withholding treatment with Sensipar® (see PRECAUTIONS).
Parathyroid Carcinoma
The recommended starting oral dose of Sensipar® is 30 mg twice daily.
The dosage of Sensipar® should be titrated every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, 90 mg twice daily, and 90 mg three or four times daily as necessary to normalize serum calcium levels.
Special Populations
Geriatric patients:
Age does not alter the pharmacokinetics of Sensipar®; no dosage adjustment is required for geriatric patients.
Patients with renal impairment:
Renal impairment does not alter the pharmacokinetics of Sensipar®; no dosage adjustment is necessary for renal impairment.
Patients with hepatic impairment:
Cinacalcet exposures, as assessed by AUC(0-inf), in patients with moderate and severe hepatic impairment (as indicated by the Child-Pugh method) were 2.4 and 4.2 times higher, respectively, than in normals. In patients with moderate and severe hepatic impairment, PTH and serum calcium concentrations should be closely monitored throughout treatment with Sensipar® (see CLINICAL PHARMACOLOGY, Pharmacokinetics and PRECAUTIONS).
Drug Interactions
Sensipar® is metabolized in part by the enzyme CYP3A4. Co-administration of ketoconazole, a strong inhibitor of CYP3A4, caused an approximate 2-fold increase in cinacalcet exposure. Dose adjustment of Sensipar® may be required and PTH and serum calcium concentrations should be closely monitored if a patient initiates or discontinues therapy with a strong CYP3A4 inhibitor (e.g., ketoconazole, erythromycin, itraconazole; see CLINICAL PHARMACOLOGY, Pharmacokinetics and PRECAUTIONS).
HOW SUPPLIED
Sensipar® 30 mg tablets are formulated as light-green, film-coated, oval-shaped tablets printed with “AMGEN” on one side and “30” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-073-30)
Sensipar® 60 mg tablets are formulated as light-green, film-coated, oval-shaped tablets printed with “AMGEN” on one side and “60” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-074-30)
Sensipar® 90 mg tablets are formulated as light-green, film-coated, oval-shaped tablets printed with “AMGEN” on one side and “90” on the opposite side, packaged in bottles of 30 tablets. (NDC 55513-075-30)
Storage
Store at 25ºC (77ºF); excursions permitted to 15-30ºC (59-86ºF). [See USP controlled room temperature].
Rx Only
This product, or its use, may be covered by one or more US Patents including US Patent Nos. 6313146, 6211244, 6031003 and 6011068, in addition to others, including patents pending.
REFERENCES
1. National Kidney Foundation: K/DOQI clinical practice guidelines: bone metabolism and disease in chronic kidney disease. American Journal of Kidney Disease 4 2:S1-S201, 2003
[Amgen Logo]
Manufactured for: Amgen
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 913201799
Issue Date: 04/2007
©2007 Amgen Inc. All rights reserved.
XXXXXXX-v2
| Sensipar (cinacalcet hydrochloride) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Sensipar (cinacalcet hydrochloride) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Sensipar (cinacalcet hydrochloride) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
Revised: 06/2007Amgen Inc.