invega (paliperidone) tablet, extended release
[ALZA Corporation]
Rx only
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks) in these subjects revealed a risk of death in the drug-treated subjects of between 1.6 to 1.7 times that seen in placebo-treated subjects. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated subjects was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. INVEGA™ (paliperidone) Extended-Release Tablets is not approved for the treatment of patients with dementia-related psychosis.
DESCRIPTION
Paliperidone, the active ingredient in INVEGA™ Extended-Release Tablets, is a psychotropic agent belonging to the chemical class of benzisoxazole derivatives. INVEGA™ contains a racemic mixture of (+)- and (-)- paliperidone. The chemical name is (±)-3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one. Its molecular formula is C23H27FN4O3 and its molecular weight is 426.49. The structural formula is:
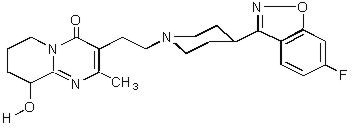
Paliperidone is sparingly soluble in 0.1N HCl and methylene chloride; practically insoluble in water, 0.1N NaOH, and hexane; and slightly soluble in N,N-dimethylformamide.
INVEGA™ (paliperidone) Extended-Release Tablets are available in 3 mg (white), 6 mg (beige), and 9 mg (pink) strengths. INVEGA™ utilizes OROS® osmotic drug-release technology (see Delivery System Components and Performance).
Inactive ingredients are carnauba wax, cellulose acetate, hydroxyethyl cellulose, propylene glycol, polyethylene glycol, polyethylene oxides, povidone, sodium chloride, stearic acid, butylated hydroxytoluene, hypromellose, titanium dioxide, and iron oxides. The 3 mg tablets also contain lactose monohydrate and triacetin.
Delivery System Components and Performance
INVEGA™ uses osmotic pressure to deliver paliperidone at a controlled rate. The delivery system, which resembles a capsule-shaped tablet in appearance, consists of an osmotically active trilayer core surrounded by a subcoat and semipermeable membrane. The trilayer core is composed of two drug layers containing the drug and excipients, and a push layer containing osmotically active components. There are two precision laser-drilled orifices on the drug-layer dome of the tablet. Each tablet strength has a different colored water-dispersible overcoat and print markings. In an aqueous environment, such as the gastrointestinal tract, the water-dispersible color overcoat erodes quickly. Water then enters the tablet through the semipermeable membrane that controls the rate at which water enters the tablet core, which, in turn, determines the rate of drug delivery. The hydrophilic polymers of the core hydrate and swell, creating a gel containing paliperidone that is then pushed out through the tablet orifices. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the stool as a tablet shell, along with insoluble core components.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Paliperidone is the major active metabolite of risperidone. The mechanism of action of paliperidone, as with other drugs having efficacy in schizophrenia, is unknown, but it has been proposed that the drug's therapeutic activity in schizophrenia is mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism.
Paliperidone is also active as an antagonist at α1 and α2 adrenergic receptors and H1 histaminergic receptors, which may explain some of the other effects of the drug. Paliperidone has no affinity for cholinergic muscarinic or β1- and β2-adrenergic receptors. The pharmacological activity of the (+)- and (-)- paliperidone enantiomers is qualitatively and quantitatively similar in vitro.
Pharmacokinetics
Following a single dose, the plasma concentrations of paliperidone gradually rise to reach peak plasma concentration (Cmax) approximately 24 hours after dosing. The pharmacokinetics of paliperidone following INVEGA™ administration are dose-proportional within the recommended clinical dose range (3 to12 mg). The terminal elimination half-life of paliperidone is approximately 23 hours.
Steady-state concentrations of paliperidone are attained within 4 – 5 days of dosing with INVEGA™ in most subjects. The mean steady-state peak:trough ratio for an INVEGA™ dose of 9 mg was 1.7 with a range of 1.2–3.1.
Following administration of INVEGA™, the (+) and (-) enantiomers of paliperidone interconvert, reaching an AUC (+) to (-) ratio of approximately 1.6 at steady state.
Absorption and Distribution
The absolute oral bioavailability of paliperidone following INVEGA™ administration is 28%.
Administration of a 12 mg paliperidone extended-release tablet to healthy ambulatory subjects with a standard high-fat/high-caloric meal gave mean Cmax and AUC values of paliperidone that were increased by 60% and 54%, respectively, compared with administration under fasting conditions. Clinical trials establishing the safety and efficacy of INVEGA™ were carried out in subjects without regard to the timing of meals. While INVEGA™ can be taken without regard to food, the presence of food at the time of INVEGA™ administration may increase exposure to paliperidone (see DOSAGE AND ADMINISTRATION).
Based on a population analysis, the apparent volume of distribution of paliperidone is 487 L. The plasma protein binding of racemic paliperidone is 74%.
Metabolism and Elimination
Although in vitrostudies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, in vivo results indicate that these isozymes play a limited role in the overall elimination of paliperidone (see PRECAUTIONS: Drug Interactions).
One week following administration of a single oral dose of 1 mg immediate-release 14C-paliperidone to 5 healthy volunteers, 59% (range 51% – 67%) of the dose was excreted unchanged into urine, 32% (26% – 41%) of the dose was recovered as metabolites, and 6% – 12% of the dose was not recovered. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the feces. Four primary metabolic pathways have been identified in vivo, none of which could be shown to account for more than 10% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission.
Population pharmacokinetic analyses found no difference in exposure or clearance of paliperidone between extensive metabolizers and poor metabolizers of CYP2D6 substrates.
Special Populations
Hepatic Impairment
In a study in subjects with moderate hepatic impairment (Child-Pugh class B), the plasma concentrations of free paliperidone were similar to those of healthy subjects, although total paliperidone exposure decreased because of a decrease in protein binding. Consequently, no dose adjustment is required in patients with mild or moderate hepatic impairment. The effect of severe hepatic impairment is unknown.
Renal Impairment
The dose of INVEGA™ should be reduced in patients with moderate or severe renal impairment (see DOSAGE AND ADMINISTRATION: Dosing in Special Populations). The disposition of a single dose paliperidone 3 mg extended-release tablet was studied in subjects with varying degrees of renal function. Elimination of paliperidone decreased with decreasing estimated creatinine clearance. Total clearance of paliperidone was reduced in subjects with impaired renal function by 32% on average in mild (CrCl = 50 to < 80 mL/min), 64% in moderate (CrCl = 30 to < 50 mL/min), and 71% in severe (CrCl = 10 to < 30 mL/min) renal impairment, corresponding to an average increase in exposure (AUCinf) of 1.5, 2.6, and 4.8 fold, respectively, compared to healthy subjects. The mean terminal elimination half-life of paliperidone was 24, 40, and 51 hours in subjects with mild, moderate, and severe renal impairment, respectively, compared with 23 hours in subjects with normal renal function (CrCl ≥ 80 mL/min).
Elderly
No dosage adjustment is recommended based on age alone. However, dose adjustment may be required because of age-related decreases in creatinine clearance (see Renal Impairment above and DOSAGE AND ADMINISTRATION: Dosing in Special Populations).
Race
No dosage adjustment is recommended based on race. No differences in pharmacokinetics were observed in a pharmacokinetic study conducted in Japanese and Caucasians.
Gender
No dosage adjustment is recommended based on gender. No differences in pharmacokinetics were observed in a pharmacokinetic study conducted in men and women.
Smoking
No dosage adjustment is recommended based on smoking status. Based on in vitro studies utilizing human liver enzymes, paliperidone is not a substrate for CYP1A2; smoking should, therefore, not have an effect on the pharmacokinetics of paliperidone.
Clinical Trials
The short-term efficacy of INVEGA™ (3 to 15 mg once daily) was established in three placebo-controlled and active-controlled (olanzapine), 6-week, fixed-dose trials in non-elderly adult subjects (mean age of 37) who met DSM-IV criteria for schizophrenia. Studies were carried out in North America, Eastern Europe, Western Europe, and Asia. The doses studied among these three trials included 3, 6, 9, 12, and 15 mg/day. Dosing was in the morning without regard to meals.
Efficacy was evaluated using the Positive and Negative Syndrome Scale (PANSS), a validated multi-item inventory composed of five factors to evaluate positive symptoms, negative symptoms, disorganized thoughts, uncontrolled hostility/excitement, and anxiety/depression. Efficacy was also evaluated using the Personal and Social Performance (PSP) scale. The PSP is a validated clinician-rated scale that measures personal and social functioning in the domains of socially useful activities (e.g., work and study), personal and social relationships, self-care, and disturbing and aggressive behaviors.
In all 3 studies (n = 1665), INVEGA™ was superior to placebo on the PANSS at all doses. Mean effects at all doses were fairly similar, although the higher doses in all studies were numerically superior. INVEGA™ was also superior to placebo on the PSP in these trials.
An examination of population subgroups did not reveal any evidence of differential responsiveness on the basis of gender, age (there were few patients over 65), or geographic region. There were insufficient data to explore differential effects based on race.
In a longer-term trial, adult outpatients meeting DSM-IV criteria for schizophrenia who had clinically responded (defined as PANSS score ≤ 70 or ≤ 4 on pre-defined PANSS subscales, as well as having been on a stable fixed dose of INVEGATM for the last two weeks of an 8-week run-in phase) were entered into a 6-week open-label stabilization phase where they received INVEGATM (doses ranging from 3 to 15 mg once daily). After the stabilization phase, patientswere randomized in a double-blind manner to either continue on INVEGATM at their achieved stable dose, or to placebo, until they experienced a relapse of schizophrenia symptoms. Relapse was pre-defined as significant increase in PANSS (or pre-defined PANSS subscales), hospitalization, clinically significant suicidal or homicidal ideation, or deliberate injury to self or others. An interim analysis of the data showed a significantly longer time to relapse in patients treated with INVEGATM compared to placebo, and the trial was stopped early because maintenance of efficacy was demonstrated.
INDICATIONS AND USAGE
INVEGA™ (paliperidone) Extended-Release Tablets is indicated for the acute and maintenance treatment of schizophrenia.
The efficacy of INVEGA™ in the acute treatment of schizophrenia was established in three 6-week, placebo-controlled, fixed-dose trials in subjects with schizophrenia.
The longer-term benefit of maintaining schizophrenic patients on monotherapy with INVEGATM after achieving a responder status for 6 weeks was demonstrated in a controlled trial (see CLINICAL PHARMACOLOGY: Clinical Trials). The physician who elects to use paliperidone for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
CONTRAINDICATIONS
INVEGA™ (paliperidone) is contraindicated in patients with a known hypersensitivity to paliperidone, risperidone, or to any components in the INVEGA™ formulation.
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. INVEGA™ (paliperidone) Extended-Release Tablets is not approved for the treatment of dementia-related psychosis (see Boxed Warning).
QT Prolongation
Paliperidone causes a modest increase in the corrected QT (QTc) interval. The use of paliperidone should be avoided in combination with other drugs that are known to prolong QTc including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval. Paliperidone should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
The effects of paliperidone on the QT interval were evaluated in a double-blind, active-controlled (moxifloxacin 400 mg single dose), multicenter QT study in adults with schizophrenia and schizoaffective disorder, and in three placebo- and active-controlled 6-week, fixed-dose efficacy trials in adults with schizophrenia.
In the QT study (n = 141), the 8 mg dose of immediate-release oral paliperidone (n=44) showed a mean placebo-subtracted increase from baseline in QTcLD of 12.3 msec (90% CI: 8.9; 15.6) on day 8 at 1.5 hours post-dose. The mean steady-state peak plasma concentration for this 8 mg dose of paliperidone immediate-release was more than twice the exposure observed with the maximum recommended 12 mg dose of INVEGA™ (Cmax ss = 113 and 45 ng/mL, respectively, when administered with a standard breakfast). In this same study, a 4 mg dose of the immediate-release oral formulation of paliperidone, for which Cmax ss = 35 ng/mL, showed an increased placebo-subtracted QTcLD of 6.8 msec (90% CI: 3.6; 10.1) on day 2 at 1.5 hours post-dose. None of the subjects had a change exceeding 60 msec or a QTcLD exceeding 500 msec at any time during this study.
For the three fixed-dose efficacy studies, electrocardiogram (ECG) measurements taken at various time points showed only one subject in the INVEGA™12 mg group had a change exceeding 60 msec at one time-point on Day 6 (increase of 62 msec). No subject receiving INVEGA™ had a QTcLD exceeding 500 msec at any time in any of these three studies.
Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including paliperidone. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases in which the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient appears to require antipsychotic drug treatment after recovery from NMS, reintroduction of drug therapy should be closely monitored, since recurrences of NMS have been reported.
Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase, but the syndrome can develop after relatively brief treatment periods at low doses, although this is uncommon.
There is no known treatment for established tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome and may thus mask the underlying process. The effect of symptomatic suppression on the long-term course of the syndrome is unknown.
Given these considerations, INVEGA™ should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that is known to respond to antipsychotic drugs. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient treated with INVEGA™, drug discontinuation should be considered. However, some patients may require treatment with INVEGA™ despite the presence of the syndrome.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with all atypical antipsychotics. These cases were, for the most part, seen in post-marketing clinical use and epidemiologic studies, not in clinical trials, and there have been few reports of hyperglycemia or diabetes in trial subjects treated with INVEGA™. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics. Because INVEGA™ was not marketed at the time these studies were performed, it is not known if INVEGA™ is associated with this increased risk.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
Gastrointestinal
Because the INVEGA™ tablet is non-deformable and does not appreciably change in shape in the gastrointestinal tract, INVEGA™ should ordinarily not be administered to patients with pre-existing severe gastrointestinal narrowing (pathologic or iatrogenic, for example: esophageal motility disorders, small bowel inflammatory disease, "short gut" syndrome due to adhesions or decreased transit time, past history of peritonitis, cystic fibrosis, chronic intestinal pseudoobstruction, or Meckel's diverticulum). There have been rare reports of obstructive symptoms in patients with known strictures in association with the ingestion of drugs in non-deformable controlled-release formulations. Because of the controlled-release design of the tablet, INVEGA™ should only be used in patients who are able to swallow the tablet whole (see PRECAUTIONS: Information for Patients).
A decrease in transit time, e.g., as seen with diarrhea, would be expected to decrease bioavailability and an increase in transit time, e.g., as seen with gastrointestinal neuropathy, diabetic gastroparesis, or other causes, would be expected to increase bioavailability. These changes in bioavailability are more likely when the changes in transit time occur in the upper GI tract.
Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients With Dementia-Related Psychosis
In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse events (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated subjects. INVEGA™ was not marketed at the time these studies were performed. INVEGA™ is not approved for the treatment of patients with dementia-related psychosis (see also Boxed WARNING, WARNINGS: Increased Mortality in Elderly Patients with Dementia-Related Psychosis).
PRECAUTIONS
General
Orthostatic Hypotension and Syncope
Paliperidone can induce orthostatic hypotension and syncope in some patients because of its alpha-blocking activity. In pooled results of the three placebo-controlled, 6-week, fixed-dose trials, syncope was reported in 0.8% (7/850) of subjects treated with INVEGA™ (3, 6, 9, 12 mg) compared to 0.3% (1/355) of subjects treated with placebo. INVEGA™ should be used with caution in patients with known cardiovascular disease (e.g., heart failure, history of myocardial infarction or ischemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications). Monitoring of orthostatic vital signs should be considered in patients who are vulnerable to hypotension.
Seizures
During premarketing clinical trials (the three placebo-controlled, 6-week, fixed-dose studies and a study conducted in elderly schizophrenic subjects), seizures occurred in 0.22% of subjects treated with INVEGA™ (3, 6, 9, 12 mg) and 0.25% of subjects treated with placebo. Like other antipsychotic drugs, INVEGA™ should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
Hyperprolactinemia
Like other drugs that antagonize dopamine D2 receptors, paliperidone elevates prolactin levels and the elevation persists during chronic administration. Paliperidone has a prolactin-elevating effect similar to that seen with risperidone, a drug that is associated with higher levels of prolactin than other antipsychotic drugs.
Hyperprolactinemia, regardless of etiology, may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously detected breast cancer. An increase in the incidence of pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats (see PRECAUTIONS: Carcinogenesis, Mutagenesis, Impairment of Fertility). Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer's dementia. INVEGA™ and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia.
Suicide
The possibility of suicide attempt is inherent in psychotic illnesses, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for INVEGA™ should be written for the smallest quantity of tablets consistent with good patient management in order to reduce the risk of overdose.
Potential for Cognitive and Motor Impairment
Somnolence and sedation were reported in subjects treated with INVEGA™ (see ADVERSE REACTIONS). Antipsychotics, including INVEGA™, have the potential to impair judgment, thinking, or motor skills. Patients should be cautioned about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that paliperidone therapy does not adversely affect them.
Priapism
Drugs with alpha-adrenergic blocking effects have been reported to induce priapism. Although no cases of priapism have been reported in clinical trials with INVEGA™, paliperidone shares this pharmacologic activity and, therefore, may be associated with this risk. Severe priapism may require surgical intervention.
Thrombotic Thrombocytopenia Purpura (TTP)
No cases of TTP were observed during clinical studies with paliperidone. Although cases of TTP have been reported in association with risperidone administration, the relationship to risperidone therapy is unknown.
Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing INVEGA™ to patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
Antiemetic Effect
An antiemetic effect was observed in preclinical studies with paliperidone. This effect, if it occurs in humans, may mask the signs and symptoms of overdosage with certain drugs or of conditions such as intestinal obstruction, Reye's syndrome, and brain tumor.
Use in Patients with Concomitant Illness
Clinical experience with INVEGA™ in patients with certain concomitant illnesses is limited (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Special Populations: Hepatic Impairment and Renal Impairment).
Patients with Parkinson's Disease or Dementia with Lewy Bodies are reported to have an increased sensitivity to antipsychotic medication. Manifestations of this increased sensitivity include confusion, obtundation, postural instability with frequent falls, extrapyramidal symptoms, and clinical features consistent with the neuroleptic malignant syndrome.
INVEGA™ has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were excluded from premarketing clinical trials. Because of the risk of orthostatic hypotension with INVEGA™, caution should be observed in patients with known cardiovascular disease (see PRECAUTIONS: General: Orthostatic Hypotension and Syncope).
Information for Patients
Physicians are advised to discuss the following issues with patients for whom they prescribe INVEGA™.
Orthostatic Hypotension
Patients should be advised that there is risk of orthostatic hypotension, particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose.
Interference With Cognitive and Motor Performance
As INVEGA™ has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that INVEGA™ therapy does not affect them adversely.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during treatment with INVEGA™.
Nursing
Patients should be advised not to breast-feed an infant if they are taking INVEGA™.
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, as there is a potential for interactions.
Alcohol
Patients should be advised to avoid alcohol while taking INVEGA™.
Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration.
Administration
Patients should be informed that INVEGA™ should be swallowed whole with the aid of liquids. Tablets should not be chewed, divided, or crushed. The medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell, along with insoluble core components, is eliminated from the body; patients should not be concerned if they occasionally notice something that looks like a tablet in their stool.
Laboratory Tests
No specific laboratory tests are recommended.
Drug Interactions
Potential for INVEGA™ to Affect Other Drugs
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with drugs that are metabolized by cytochrome P450 isozymes. In vitro studies in human liver microsomes showed that paliperidone does not substantially inhibit the metabolism of drugs metabolized by cytochrome P450 isozymes, including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. Therefore, paliperidone is not expected to inhibit clearance of drugs that are metabolized by these metabolic pathways in a clinically relevant manner. Paliperidone is also not expected to have enzyme inducing properties.
At therapeutic concentrations, paliperidone did not inhibit P-glycoprotein. Paliperidone is therefore not expected to inhibit P-glycoprotein-mediated transport of other drugs in a clinically relevant manner.
Given the primary CNS effects of paliperidone (see ADVERSE REACTIONS), INVEGA™ should be used with caution in combination with other centrally acting drugs and alcohol. Paliperidone may antagonize the effect of levodopa and other dopamine agonists.
Because of its potential for inducing orthostatic hypotension,an additive effect may be observed when INVEGA™ is administered with other therapeutic agents that have this potential (see PRECAUTIONS: General: Orthostatic Hypotension and Syncope).
Potential for Other Drugs to Affect INVEGA™
Paliperidone is not a substrate of CYP1A2, CYP2A6, CYP2C9, and CYP2C19, so that an interaction with inhibitors or inducers of these isozymes is unlikely. While in vitro studies indicate that CYP2D6 and CYP3A4 may be minimally involved in paliperidone metabolism, in vivo studies do not show decreased elimination by these isozymes and they contribute to only a small fraction of total body clearance.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of paliperidone have not been performed.
Carcinogenicity studies of risperidone, which is extensively converted to paliperidone in rats, mice, and humans, were conducted in Swiss albino mice and Wistar rats. Risperidone was administered in the diet at daily doses of 0.63, 2.5, and 10 mg/kg for 18 months to mice and for 25 months to rats. A maximum tolerated dose was not achieved in male mice. There were statistically significant increases in pituitary gland adenomas, endocrine pancreas adenomas, and mammary gland adenocarcinomas. The no-effect dose for these tumors was less than or equal to the maximum recommended human dose of risperidone on a mg/m2 basis (see risperidone package insert). An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be mediated by prolonged dopamine D2 antagonism and hyperprolactinemia. The relevance of these tumor findings in rodents in terms of human risk is unknown (see PRECAUTIONS: General: Hyperprolactinemia).
Mutagenesis
No evidence of genotoxic potential for paliperidone was found in the Ames reverse mutation test, the mouse lymphoma assay, or the in vivo rat micronucleus test.
Impairment of Fertility
In a study of fertility, the percentage of treated female rats that became pregnant was not affected at oral doses of paliperidone of up to 2.5 mg/kg/day. However, pre- and post-implantation loss was increased, and the number of live embryos was slightly decreased, at 2.5 mg/kg, a dose that also caused slight maternal toxicity. These parameters were not affected at a dose of 0.63 mg/kg, which is half of the maximum recommended human dose on a mg/m2 basis.
The fertility of male rats was not affected at oral doses of paliperidone of up to 2.5 mg/kg/day, although sperm count and sperm viability studies were not conducted with paliperidone. In a subchronic study in Beagle dogs with risperidone, which is extensively converted to paliperidone in dogs and humans, all doses tested (0.31–5.0 mg/kg) resulted in decreases in serum testosterone and in sperm motility and concentration. Serum testosterone and sperm parameters partially recovered, but remained decreased after the last observation (two months after treatment was discontinued).
Pregnancy
Pregnancy Category C
In studies in rats and rabbits in which paliperidone was given orally during the period of organogenesis, there were no increases in fetal abnormalities up to the highest doses tested (10 mg/kg/day in rats and 5 mg/kg/day in rabbits, which are 8 times the maximum recommended human dose on a mg/m2 basis).
In rat reproduction studies with risperidone, which is extensively converted to paliperidone in rats and humans, increases in pup deaths were seen at oral doses which are less than the maximum recommended human dose of risperidone on a mg/m2 basis (see risperidone package insert).
Use of first generation antipsychotic drugs during the last trimester of pregnancy has been associated with extrapyramidal symptoms in the neonate. These symptoms are usually self-limited. It is not known whether paliperidone, when taken near the end of pregnancy, will lead to similar neonatal signs and symptoms.
There are no adequate and well controlled studies of INVEGA™ in pregnant women. INVEGA™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
The effect of INVEGA™ on labor and delivery in humans is unknown.
Nursing Mothers
In animal studies with paliperidone and in human studies with risperidone, paliperidone was excreted in the milk.Therefore, women receiving INVEGA™ should not breast-feed infants.
Pediatric Use
Safety and effectiveness of INVEGA™ in patients< 18 years of age have not been established.
Geriatric Use
The safety, tolerability, and efficacy of INVEGA™ were evaluated in a 6-week placebo-controlled study of 114 elderly subjects with schizophrenia (65 years of age and older, of whom 21 were 75 years of age and older). In this study, subjects received flexible doses of INVEGA™ (3 to 12 mg once daily). In addition, a small number of subjects 65 years of age and older were included in the 6-week placebo-controlled studies in which adult schizophrenic subjects received fixed doses of INVEGA™ (3 to 15 mg once daily, see CLINICAL PHARMACOLOGY: Clinical Trials).
Overall, of the total number of subjects in clinical studies of INVEGA™ (n = 1796), including those who received INVEGA™ or placebo, 125 (7.0%) were 65 years of age and older and 22 (1.2%) were 75 years of age and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney and clearance is decreased in patients with moderate to severe renal impairment (see CLINICAL PPHARMACOLOGY: Pharmacokinetics: Special Populations: Renal Impairment), who should be given reduced doses. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see DOSAGE AND ADMINISTRATION: Dosing in Special Populations).
ADVERSE REACTIONS
The information below is derived from a clinical trial database for INVEGA™ consisting of 2720 patients and/or normal subjects exposed to one or more doses of INVEGA™ for the treatment of schizophrenia.
Of these 2720 patients, 2054 were patients who received INVEGA™ while participating in multiple dose, effectiveness trials. The conditions and duration of treatment with INVEGA™ varied greatly and included (in overlapping categories) open-label and double-blind phases of studies, inpatients and outpatients, fixed-dose and flexible-dose studies, and short-term and longer-term exposure. Adverse events were assessed by collecting adverse events and performing physical examinations, vital signs, weights, laboratory analyses and ECGs.
Adverse events during exposure were obtained by general inquiry and recorded by clinical investigators using their own terminology. Consequently, to provide a meaningful estimate of the proportion of individuals experiencing adverse events, events were grouped in standardized categories using MedDRA terminology.
The stated frequencies of adverse events represent the proportions of individuals who experienced a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
The information presented in these sections was derived from pooled data from the three placebo-controlled, 6-week, fixed-dose studies based on subjects with schizophrenia who received INVEGATM at daily doses within the recommended range of 3 to 12 mg (n = 850). Additional safety information from the placebo-controlled phase of the long-term maintenance study, in which subjects received INVEGATM at daily doses within the range of 3 to 15 mg (n = 104), is also included.
Adverse Events Observed in Short-Term, Placebo-Controlled Trials of Subjects with Schizophrenia
Adverse Events Occurring at an Incidence of 2% or More Among INVEGA™Treated Patients with Schizophrenia and More Frequent on Drug than Placebo
Table 1 enumerates the pooled incidences of treatment-emergent adverse events that were spontaneously reported in the three placebo-controlled, 6-week, fixed-dose studies, listing those events that occurred in 2% or more of subjects treated with INVEGA™ in any of the dose groups, and for which the incidence in INVEGA™ -treated subjects in any of the dose groups was greater than the incidence in subjects treated with placebo.
| Percentage of Patients Reporting Event | |||||
|---|---|---|---|---|---|
| INVEGA™ | |||||
| Body System or Organ Class Dictionary-derived Term |
Placebo (N=355) |
3 mg once daily (N=127) |
6 mg once daily (N=235) |
9 mg once daily (N=246) |
12 mg once daily (N=242) |
|
|||||
| Percentage of subjects with adverse events | 66 | 72 | 66 | 70 | 76 |
| Cardiac disorders | |||||
| Atrioventricular block first degree | 1 | 2 | 0 | 2 | 1 |
| Bundle branch block | 2 | 3 | 1 | 3 | <1 |
| Sinus arrhythmia | 0 | 2 | 1 | 1 | <1 |
| Tachycardia | 7 | 14 | 12 | 12 | 14 |
| Eye disorders | |||||
| Vision blurred | 1 | 1 | <1 | 0 | 2 |
| Gastrointestinal disorders | |||||
| Abdominal pain upper | 1 | 1 | 3 | 2 | 2 |
| Dry mouth | 1 | 2 | 3 | 1 | 3 |
| Dyspepsia | 4 | 2 | 3 | 2 | 5 |
| Nausea | 5 | 6 | 4 | 4 | 4 |
| Salivary hypersecretion | <1 | 0 | <1 | 1 | 4 |
| General disorders | |||||
| Asthenia | 1 | 2 | <1 | 2 | 2 |
| Fatigue | 1 | 2 | 1 | 2 | 2 |
| Pyrexia | 1 | 1 | <1 | 2 | 2 |
| Investigations | |||||
| Blood insulin increased | 1 | 2 | 1 | 1 | <1 |
| Blood pressure increased | 1 | 2 | <1 | <1 | 1 |
| Electrocardiogram QT corrected interval prolonged | 3 | 3 | 4 | 3 | 5 |
| Electrocardiogram T wave abnormal | 1 | 2 | 1 | 2 | 1 |
| Musculoskeletal and connective tissue disorders | |||||
| Back pain | 1 | 1 | 1 | 1 | 2 |
| Pain in extremity | 1 | 0 | 1 | 0 | 2 |
| Nervous system disorders | |||||
| Akathisia | 4 | 4 | 3 | 8 | 10 |
| Dizziness | 4 | 6 | 5 | 4 | 5 |
| Dystonia | 1 | 1 | 1 | 5 | 4 |
| Extrapyramidal disorder | 2 | 5 | 2 | 7 | 7 |
| Headache | 12 | 11 | 12 | 14 | 14 |
| Hypertonia | 1 | 2 | 1 | 4 | 3 |
| Parkinsonism | 0 | 0 | <1 | 2 | 1 |
| Somnolence | 7 | 6 | 9 | 10 | 11 |
| Tremor | 3 | 3 | 3 | 4 | 3 |
| Psychiatric disorders | |||||
| Anxiety | 8 | 9 | 7 | 6 | 5 |
| Respiratory, thoracic and mediastinal disorders | |||||
| Cough | 1 | 3 | 2 | 3 | 2 |
| Vascular disorders | |||||
| Orthostatic hypotension | 1 | 2 | 1 | 2 | 4 |
Dose-Related Adverse Events in Clinical Trials
Based on the pooled data from the three placebo-controlled, 6-week, fixed-dose studies, adverse events that occurred with a greater than 2% incidence in the subjects treated with INVEGA™, the incidences of the following adverse events increased with dose: somnolence, orthostatic hypotension, salivary hypersecretion, akathisia, dystonia, extrapyramidal disorder, hypertonia and Parkinsonism. For most of these, the increased incidence was seen primarily at the 12 mg, and in some cases the 9 mg dose.
Common and Drug-Related Adverse Events in Clinical Trials
In the pooled data from the three placebo-controlled, 6–week, fixed-dose studies, adverse events reported in 5% or more of subjects treated with INVEGA™ and at least twice the placebo rate for at least one dose included: akathisia and extrapyramidal disorder.
Extrapyramidal Symptoms (EPS) in Clinical Trials
Pooled data from the three placebo-controlled, 6-week, fixed-dose studies provided information regarding treatment-emergent EPS. Several methods were used to measure EPS: (1) the Simpson-Angus global score (mean change from baseline) which broadly evaluates Parkinsonism, (2) the Barnes Akathisia Rating Scale global clinical rating score (mean change from baseline) which evaluates akathisia, (3) use of anticholinergic medications to treat emergent EPS, and (4) incidence of spontaneous reports of EPS. For the Simpson-Angus Scale, spontaneous EPS reports and use of anticholinergic medications, there was a dose-related increase observed for the 9 mg and 12 mg doses. There was no difference observed between placebo and INVEGATM 3 mg and 6 mg doses for any of these EPS measures.
| Percentage of Patients | |||||
|---|---|---|---|---|---|
| INVEGA™ | |||||
| EPS Group | Placebo (N=355) |
3 mg once daily (N=127) |
6 mg once daily (N=235) |
9 mg once daily (N=246) |
12 mg once daily (N=242) |
|
|||||
| Parkinsonism * | 9 | 11 | 3 | 15 | 14 |
| Akathisia † | 6 | 6 | 4 | 7 | 9 |
| Use of anticholinergic medications ‡ | 10 | 10 | 9 | 22 | 22 |
| Percentage of Patients | |||||
|---|---|---|---|---|---|
| INVEGA™ | |||||
| EPS Group | Placebo (N=355) |
3 mg once daily (N=127) |
6 mg once daily (N=235) |
9 mg once daily (N=246) |
12 mg once daily (N=242) |
| Dyskinesia group includes: Dyskinesia, Extrapyramidal
disorder, Muscle twitching, Tardive dyskinesia Dystonia group includes: Dystonia, Muscle spasms, Oculogyration, Trismus Hyperkinesia group includes: Akathisia, Hyperkinesia Parkinsonism group includes: Bradykinesia, Cogwheel rigidity, Drooling, Hypertonia, Hypokinesia, Muscle rigidity, Musculoskeletal stiffness, Parkinsonism Tremor group includes: Tremor |
|||||
| Overall percentage of patients with EPS-related AE |
11.0 | 12.6 | 10.2 | 25.2 | 26.0 |
| Dyskinesia | 3.4 | 4.7 | 2.6 | 7.7 | 8.7 |
| Dystonia | 1.1 | 0.8 | 1.3 | 5.3 | 4.5 |
| Hyperkinesia | 3.9 | 3.9 | 3.0 | 8.1 | 9.9 |
| Parkinsonism | 2.3 | 3.1 | 2.6 | 7.3 | 6.2 |
| Tremor | 3.4 | 3.1 | 2.6 | 4.5 | 3.3 |
Adverse Events Associated with Discontinuation of Treatment in Controlled Clinical Studies
Based on the pooled data from the three placebo-controlled, 6–week, fixed-dose studies, there was no difference in the incidence of discontinuation due to adverse events between INVEGA™-treated (5%) and placebo-treated (5%) subjects. The types of adverse events that led to discontinuation were similar for the INVEGA™-and placebo-treated subjects, except for Nervous System Disorders events which were more common among INVEGA™-treated subjects than placebo-treated subjects (2% and 0%, respectively), and Psychiatric Disorders events which were more common among placebo-treated subjects than INVEGA™-treated subjects (3% and 1%, respectively).
Demographic Differences in Adverse Reactions in Clinical Trials
An examination of population subgroups in the three placebo-controlled, 6-week, fixed-dose studies did not reveal any evidence of differences in safety on the basis of age, gender or race (see PRECAUTIONS: Geriatric Use).
Laboratory Test Abnormalities in Clinical Trials
In the pooled data from the three placebo-controlled, 6-week, fixed-dose studies, between-group comparisons revealed no medically important differences between INVEGA™ and placebo in the proportions of subjects experiencing potentially clinically significant changes in routine hematology, urinalysis, or serum chemistry, including mean changes from baseline in fasting glucose, insulin, c-peptide, triglyceride, HDL, LDL, and total cholesterol measurements. Similarly, there were no differences between INVEGA™ and placebo in the incidence of discontinuations due to changes in hematology, urinalysis, or serum chemistry. However, INVEGA™ was associated with increases in serum prolactin (see PRECAUTIONS: General: Hyperprolactinemia).
Weight Gain in Clinical Trials
In the pooled data from the three placebo-controlled, 6-week, fixed-dose studies, the proportions of subjects having a weight gain of ≥ 7% of body weight were similar for INVEGA™ 3 mg and 6 mg (7% and 6%, respectively) and placebo (5%), but there was a higher incidence of weight gain for INVEGA™ 9 mg and 12 mg (9% and 9%, respectively).
Other Events Observed During the Premarketing Evaluation of INVEGA™
The following list contains all serious and non-serious treatment-emergent adverse events reported at any time by individuals taking INVEGA™ during any phase of a trial within the premarketing database (n = 2720), except (1) those listed in Table 1 above or elsewhere in labeling, (2) those for which a causal relationship to INVEGA™ use was considered remote, and (3) those occurring in only one subject treated with INVEGA™ and that were not acutely life-threatening.
Events are classified within body system categories using the following definitions: very frequent adverse events are defined as those occurring on one or more occasions in at least 1/10 subjects, frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 subjects, infrequentadverse events are those occurring on one or more occasions in 1/100 to 1/1000 subjects, and rare events are those occurring on one or more occasions in less than 1/1000 subjects.
Blood and Lymphatic System Disorders: rare: thrombocytopenia
Cardiac Disorders: frequent:palpitations; infrequent: bradycardia
Gastrointestinal Disorders:frequent: abdominal pain; infrequent: swollen tongue
General Disorders: infrequent: edema
Immune Disorder: rare: anaphylactic reaction
Nervous System Disorders:rare: coordination abnormal
Psychiatric Disorders: infrequent: confusional state
Respiratory, Thoracic and Mediastinal Disorders: frequent:dyspnea; rare: pulmonary embolus
Vascular Disorders: rare: ischemia, venous thrombosis
The safety of INVEGATM was also evaluated in a long-term trial designed to assess the maintenance of effect with INVEGATM in adults with schizophrenia (see CLINICAL PHARMACOLOGY: Clinical Trials). In general, adverse event types, frequencies, and severities during the initial 14-week open-label phase of this study were comparable to those observed in the 6-week, placebo-controlled, fixed-dose studies. Adverse events reported during the long-term double-blind phase of this study were similar in type and severity to those observed in the initial 14-week open-label phase.
Adverse Events Reported With Risperidone
Paliperidone is the major active metabolite of risperidone. Adverse events reported with risperidone can be found in the ADVERSE REACTIONS section of the risperidone package insert.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
INVEGA™ (paliperidone) is not a controlled substance.
Physical and Psychological Dependence
Paliperidone has not been systematically studied in animals or humans for its potential for abuse, tolerance, or physical dependence. It is not possible to predict the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of INVEGATM misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
OVERDOSAGE
Human Experience
While experience with paliperidone overdose is limited, among the few cases of overdose reported in pre-marketing trials, the highest estimated ingestion of INVEGA™ was 405 mg. Observed signs and symptoms included extrapyramidal symptoms and gait unsteadiness. Other potential signs and symptoms include those resulting from an exaggeration of paliperidone's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and QT prolongation.
Paliperidone is the major active metabolite of risperidone. Overdose experience reported with risperidone can be found in the OVERDOSAGE section of the risperidone package insert.
Management of Overdosage
There is no specific antidote to paliperidone, therefore, appropriate supportive measures should be instituted and close medical supervision and monitoring should continue until the patient recovers. Consideration should be given to the extended-release nature of the product when assessing treatment needs and recovery. Multiple drug involvement should also be considered.
In case of acute overdose, establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation if patient is unconscious) and administration of activated charcoal together with a laxative should be considered.
The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
Cardiovascular monitoring should commence immediately, including continuous electrocardiographic monitoring for possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with an acute overdose of paliperidone. Similarly the alpha-blocking properties of bretylium might be additive to those of paliperidone, resulting in problematic hypotension.
Hypotension and circulatory collapse should be treated with appropriate measures, such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of paliperidone-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered.
DOSAGE AND ADMINISTRATION
The recommended dose of INVEGA™ (paliperidone) Extended-Release Tablets is 6 mg once daily, administered in the morning. Initial dose titration is not required. Although it has not been systematically established that doses above 6 mg have additional benefit, there was a general trend for greater effects with higher doses. This must be weighed against the dose-related increase in adverse effects. Thus, some patients may benefit from higher doses, up to 12 mg/day, and for some patients, a lower dose of 3 mg/day may be sufficient. Dose increases above 6 mg/day should be made only after clinical reassessment and generally should occur at intervals of more than 5 days. When dose increases are indicated, small increments of 3 mg/day are recommended. The maximum recommended dose is 12 mg/day.
INVEGATM can be taken with or without food. Clinical trials establishing the safety and efficacy of INVEGATM were carried out in patients without regard to food intake.
INVEGA™ must be swallowed whole with the aid of liquids. Tablets should not be chewed, divided, or crushed. The medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell, along with insoluble core components, is eliminated from the body; patients should not be concerned if they occasionally notice in their stool something that looks like a tablet.
Concomitant use of INVEGA™ with risperidone has not been studied. Since paliperidone is the major active metabolite of risperidone, consideration should be given to the additive paliperidone exposure if risperidone is coadministered with INVEGA™.
In a longer-term study, INVEGATM has been shown to be effective in delaying time to relapse in patients with schizophrenia who were stabilized on INVEGATM for 6 weeks (see CLINICAL PHARMACOLOGY: Clinical Trials). INVEGATM should be prescribed at the lowest effective dose for maintaining clinical stability and the physician should periodically reevaluate the long-term usefulness of the drug in individual patients.
Dosing in Special Populations
Hepatic Impairment
For patients with mild to moderate hepatic impairment, (Child-Pugh Classification A and B), no dose adjustment is recommended (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Special Populations: Hepatic Impairment).
Renal Impairment
Dosing must be individualized according to the patient's renal function status. For patients with mild renal impairment (creatinine clearance ≥ 50 to < 80 mL/min), the maximum recommended dose is 6 mg once daily. For patients with moderate to severe renal impairment (creatinine clearance 10 to < 50 mL/min), the maximum recommended dose of INVEGA™ is 3 mg once daily.
Elderly
Because elderly patients may have diminished renal function, dose adjustments may be required according to their renal function status. In general, recommended dosing for elderly patients with normal renal function is the same as for younger adult patients with normal renal function. For patients with moderate to severe renal impairment (creatinine clearance 10 to < 50 mL/min) the maximum recommended dose of INVEGA™ is 3 mg once daily (see Renal Impairment above).
HOW SUPPLIED
INVEGA™ (paliperidone) Extended-Release Tablets are available in the following strengths and packages. All tablets are capsule-shaped.
3 mg tablets are white and imprinted with "PALI 3", and are available in bottles of 30 (NDC 50458-550-01), bottles of 350 (NDC 50458-550-02), and hospital unit dose packs of 100 (NDC 50458-550-10).
6 mg tablets are beige and imprinted with "PALI 6", and are available in bottles of 30 (NDC 50458-551-01), bottles of 350 (NDC 50458-551-02), and hospital unit dose packs of 100 (NDC 50458-551-10).
9 mg tablets are pink and imprinted with "PALI 9", and are available in bottles of 30 (NDC 50458-552-01), bottles of 350 (NDC 50458-552-02), and hospital unit dose packs of 100 (NDC 50458-552-10).
Storage
Store up to 25°C (77°F); excursions permitted to 15 – 30°C (59 – 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
Keep out of reach of children.
INVEGA™ (paliperidone) Extended-Release Tablets
Manufactured by:
ALZA Corporation
Mountain
View, CA 94043
Distributed by:
Janssen, L.P.
Titusville, NJ 08560
OROS® is a registered trademark of ALZA Corporation
April
2007
10105901
| INVEGA (paliperidone) | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| INVEGA (paliperidone) | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| INVEGA (paliperidone) | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Revised: 05/2007ALZA Corporation