SENNA
-
docusate sodium tablet
NCS HealthCare of KY, Inc dba Vangard Labs
----------
Senna PlusActive ingredient(s)
Sennosides from Senna Concentrate 8.6mg
Docusate Sodium 50mg
Purpose
Laxetive
Stool Softener
Use(s)
-fort the short term relief of constipation
-products bowel movement in 6 to 12 hours
Warnings
Contains FD&C Yellow #5 Lake (Tartrazine) as a color additive
Do not use
-for longer than one week
-when abdominal pain, neusea, or vomiting are present
Ask a doctor or pharmacist before use if
-you have noticed a sudden change in bowel habits that lasts over a period of two weeks
Stop use and ask a doctor if
-you have rectal bleeding
-you fail to have a bowel movement after use of this product, this may indicate a serious condition
Pregnancy/Breastfeeding
ask a health care professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Take preferrably at bedtime or as directed by a doctor
-If you do not have a comfortable bowel movement by the second day, increase dose by one tablet (do not exceed the maximum dosage or decrease dose until you are comfortable
age starting dosage maximum dosage
-adults and children over 12 years 2 tablets once a day 4 tablets twice a day
-children 6 to 12 years 1 tablet once a day 2 tablets twice a day
-children 2 to under 6 years 1/2 tablet once a day 1 tablet twice a day
-children under 2 years do not use
Storage
Store at room temperature, USP
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, FD&C Yellow #5 Lake, FD&C Yellow #6 Lake, Hypromellose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Polyethylene Glycol, Sodium Benzoate, Starch, Stearic Acid and Titanium Dioxide.
Questions
Serious Adverse Effects Call: (800) 616-2471
This product is not manufactured or distributed by Purdue Pharma L.P. owner of the registered trademark Senokto-S
Distributed by: Major Pharmaceuticals 31778 Enterprise Drive, Livonia, MI 48150
Rev. 02/08
M-29
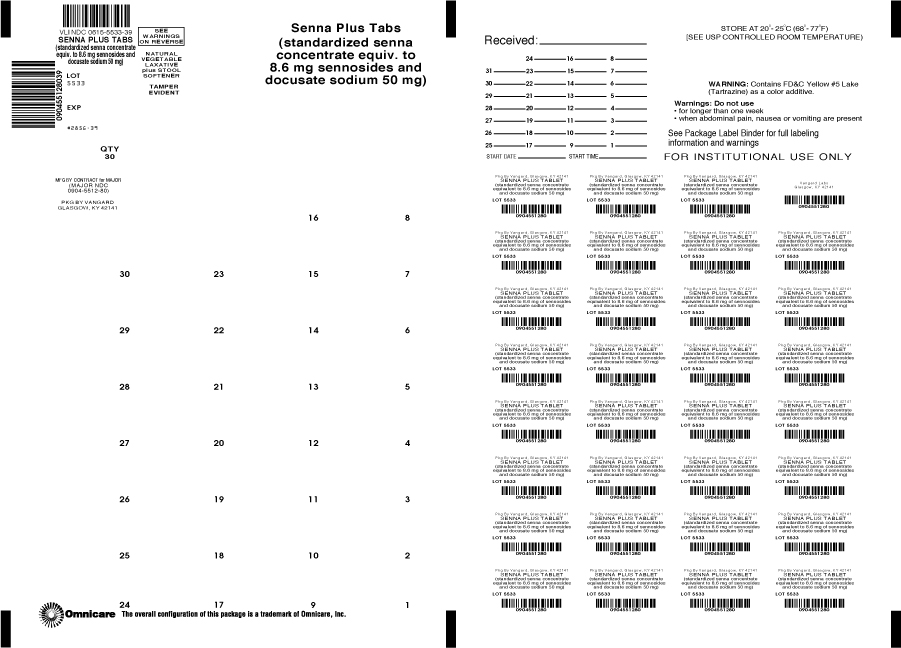
Principal Display Panel
Senna Plus Tablets
(Standardized senna concentrate equiv. to
8.6mg sennosides and docusate sodium 50mg)
| SENNA
senna plus tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part334 | 07/01/2010 | |
| Labeler - NCS HealthCare of KY, Inc dba Vangard Labs (050052943) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| NCS HealthCare of KY, Inc dba Vangard Labs | 050052943 | RELABEL, REPACK | |