VICKS NYQUIL SINEX
-
acetaminophen,
doxylamine succinate and
phenylephrine hydrochloride capsule, liquid filled
The Procter & Gamble Manufacturing Company
----------
NyQuil®Sinex®
Drug Facts
| Active ingredients (in each LiquiCap) | Purpose |
|---|---|
| Acetaminophen 325 mg | Pain reliever |
| Doxylamine succinate 6.25 mg | Antihistamine |
| Phenylephrine HCl 5 mg | Nasal decongestant |
Uses
temporarily relieves nasal & sinus symptoms:
- sinus pain
- headache
- nasal & sinus congestion
- runny nose & sneezing
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4 doses in 24 hrs, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- to make a child sleep
Ask a doctor before use if you have
- liver disease
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives & tranquilizers may increase drowsiness
Stop use and ask a doctor if
- redness or swelling is present
- you get nervous, dizzy or sleepless
- fever gets worse or lasts more than 3 days
- new symptoms occur
- symptoms do not get better within 7 days or are accompanied by a fever
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Overdose warning
Taking more than directed can cause serious health problems. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults & for children even if you do not notice any signs or symptoms.
Directions
- take only as directed - see Overdose warning
- do not exceed 4 doses per 24 hrs
| adults & children 12 yrs & over | 2 LiquiCaps with water every 4 hrs |
| children 4 to under 12 yrs | ask a doctor |
| children under 4 yrs | do not use |
- when using other DayQuil® or NyQuil products, carefully read each label to insure correct dosing
Other information
- store at room temperature
Inactive ingredients
FD&C Blue No. 1, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol special, titanium dioxide
Questions?
1-800-362-1683
www.vicks.com
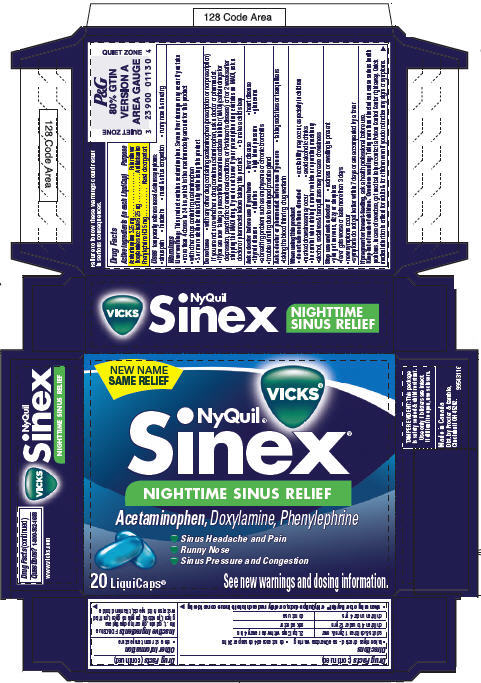
PRINCIPAL DISPLAY PANEL - 20 LiquiCaps Carton
NEW NAME
SAME RELIEF
VICKS®
NyQuil®
Sinex®
NIGHTTIME SINUS RELIEF
Acetaminophen, Doxylamine, Phenylephrine
- Sinus Headache and Pain
- Runny Nose
- Sinus Pressure and Congestion
20 LiquiCaps®
See new warnings and dosing information.
| VICKS NYQUIL SINEX
acetaminophen, doxylamine succinate, and phenylephrine hydrochloride capsule, liquid filled |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 07/06/2010 | |
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |