sodium chloride (Sodium Chloride) solution
[Mallinckrodt Inc. tyco Healthcare]
August 2006
PRESCRIBING INFORMATION
Rx only
Mallinckrodt Inc.
For Intravascular Use Only
DESCRIPTION
Sodium Chloride Injection, USP 0.9% is a formulation of sodium chloride in Water for Intravascular Injection. No preservative, antimicrobial agent or buffer is added. Sodium Chloride Injection, USP 0.9% is provided as a sterile, nonpyrogenic, clear, colorless, odorless solution.
Each mL of Sodium Chloride Injection, USP 0.9% contains 9 mg of sodium chloride. The pH is 4.5 to 7.0. The osmolarity is 308 mOsm/L (calc.). Sodium Chloride Injection, USP 0.9% is designated chemically as sodium chloride and its molecular formula is NaCl. Its molecular weight is 58.44.
Sodium Chloride Injection, USP 0.9% is provided in a 50 mL syringe with a 50 mL fill and a 125 mL syringe with a 125 mL fill. The syringes are for single patient use and are disposable and not meant for reuse.
CLINICAL PHARMACOLOGY
Sodium Chloride Injection, USP 0.9% has approximately the same osmotic pressure as plasma.
INDICATIONS AND USAGE
50 mL Syringe
Sodium Chloride Injection, USP 0.9% is indicated for use in flushing compatible contrast agents through Mallinckrodt intravenous administration sets into indwelling intravascular access devices when delivered manually or by Mallinckrodt Optistar® LE power injector.
125 mL Syringe
Sodium Chloride Injection, USP 0.9% is indicated for use in flushing compatible contrast agents through Mallinckrodt intravenous administration sets into indwelling intravascular access devices only when delivered by the following Mallinckrodt power injectors: Angiomat® Illumena®, CT9000®, CT9000® ADV, Optistat® and OptiVantage™ DH.
CONTRAINDICATIONS
None known.
WARNINGS AND PRECAUTIONS
Air Embolism
REMOVE ALL AIR FROM THE SYRINGE AND ASSOCIATED TUBING PRIOR TO INJECTION TO AVOID AIR EMBOLUS WITH THE ASSOCIATED RISK OF STROKE, ORGAN ISCHEMIA AND/OR INFARCTION, AND DEATH.
Infectious Complications
Use aseptic technique. Inspect the syringe for signs of break in sterility. Do not use if the syringe shows signs of damage, leakage or a loose fitting tip cover (see DOSAGE AND ADMINISTRATION, Assembly and Inspection). Do not use if the solution is cloudy or discolored or contains particulate matter. Use of a damaged syringe or failure to maintain aseptic technique may result in infection, sepsis and death.
Fluid Overload
Sodium Chloride Injection, USP 0.9% should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states with edema, sodium retention, or hypernatremia.
Each patient's age, body weight, fluid status, concomitant medical conditions and planned radiological procedure should be taken into account to determine if use of Sodium Chloride Injection, USP 0.9% is appropriate for them.
Extravasation
Mechanical compression of neurovascular structures may result from extravasation of the normal saline. Extravasation of contrast agent may result in tissue injury by osmolar and direct cytotoxicity (see package inserts of specific contrast agents). Intravascular catheter patency must be established prior to the administration of Sodium Chloride Injection, USP 0.9%.
Do not reuse. For single patient use only. Do not use if the syringe, piston, or tip cap are damaged in any way. Do not use if the tip cap is loose or if there are signs of leakage.
Pregnancy Category C
Animal reproductive studies have not been conducted with Sodium Chloride Injection, USP 0.9%. It is also not known whether Sodium Chloride Injection, USP 0.9% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Chloride Injection, USP 0.9% should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and effectiveness of Sodium Chloride Injection, USP 0.9% administered by power injection in pediatric patients have not been established. Administration of Sodium Chloride Injection, USP 0.9% to pediatric patients by power injection is not recommended. Manual injection of Sodium Chloride Injection, USP 0.9% to pediatric patients should take into account the patient's weight, fluid status, and concomitant medical conditions to determine if use of Sodium Chloride Injection, USP 0.9% is appropriate.
The safety of manual injection of Sodium Chloride Injection, USP 0.9% in pediatric patients is supported by reported clinical experience with intravenous infusion and flush of sodium chloride injection in pediatric patients.
To minimize the risk of fluid overload, the smallest dose of Sodium Chloride Injection, USP 0.9% necessary for manually flushing contrast agent through the vascular access line should be used.
Geriatric Use
No clinical studies of Sodium Chloride Injection, USP 0.9% were conducted. Other reported clinical experience with sodium chloride injection has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
There may be reactions due to solution or technique of administration which include air embolization with stroke, chest pain, and dyspnea, arrhythmia, hypotension, myocardial infarction, sepsis, febrile response, local tenderness, infection at the site of injection, venous thrombosis or phlebitis extending from injection site, extravasation, fluid overload, and hypervolemia.
OVERDOSAGE
Use of Sodium Chloride Injection, USP 0.9% may pose a threat of overdose marked by electrolyte disturbance and/or fluid overload, particularly in pediatric patients and patients with compromised renal or cardiac function. In the event this should occur, discontinue the infusion, reevaluate the patient and institute appropriate corrective action.
DOSAGE AND ADMINISTRATION
The 50 mL syringe and the 125 mL syringe are intended for Single Patient Use.
The volume of the saline flush should be determined for each patient individually based, in part, on the imaging procedure, the location of the vascular access device, the length of tubing between the Mallinckrodt contrast agent power injector and the vascular access device and the recommendations made on the package insert for the contrast agent. Typical Sodium Chloride Injection, USP 0.9% flush volumes following contrast agent administration in adults are 10 to 25 mL per injection at rates not to exceed 10 mL/sec.
Use of some Mallinckrodt contrast agent power injectors allows for additional infusion of Sodium Chloride Injection, USP 0.9% to maintain the patency of vascular access. Typical infusion rates used for this purpose are in the range of 0.5 to 1 mL per minute.
Infusion rates and flush volumes should be individualized for each patient based on their body weight, fluid status and concomitant medical conditions.
Consult the Mallinckrodt contrast agent power injector manual for proper use.
Drug Handling
EXPEL AIR BEFORE USE.
Do not use if packaging is damaged, wet or not intact. Do not use if the syringe or its cap is damaged or displaced, or if any leakage is evident. Do not use if solution is hazy, cloudy, discolored or contains particulate matter.
Use aseptic technique.
Residual air in both the syringe and tubing should be expelled prior to connection with the patient's vascular access.
Instructions for assembly and inspection of the Sodium Chloride Injection, USP 0.9% syringes prior to use are printed on this sheet.
HOW SUPPLIED
Sodium Chloride Injection, USP 0.9% is a clear, colorless, odorless solution containing 0.9 mg/mL of sodium chloride. Sodium Chloride Injection, USP 0.9% is supplied in 50 and 125 mL syringes containing 50 and 125 mL of solution respectively. Each syringe is sealed with rubber closures and the contents are sterile. The 125 mL syringe is supplied with a luer locknut adapter which is cleared for manufacture and distribution as a device under 510K 862653. The syringes are contained in shipping cartons with the following configurations:
50 mL in plastic syringes in cartons of 10 syringes
(NDC Code 0019-1188-75)
125 mL in plastic syringes in cartons of 20 syringes
(NDC Code 0019-1188-81)
Storage: Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [See USP Controlled Room Temperature].
PROTECT FROM FREEZING
Optistat and OptiVantage are trademarks of Mallinckrodt Inc.
Optistar, Angiomat, Illumena, and CT9000 are registered trademarks of Liebel-Flarsheim Company.
tyco/Healthcare logo
Manufactured and Distributed by:
Mallinckrodt Inc. tyco Healthcare
St. Louis, MO 63042
www.Mallinckrodt.com
MKR 1188806
Rev 8/06
Printed in U.S.A.
50 mL Syringe:
| Assembly and Inspection | NOTE: Exterior of syringe is not sterile. |
|
Contents of syringe and area under tip cap and piston ribs are sterile and should be treated accordingly. |
|
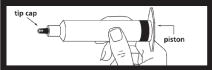 | Remove syringe from carton and inspect the area around the tip cap and outside of piston for signs of leakage. Do not use if leakage is observed. |
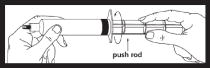 | After screwing the push rod into the syringe piston, it is important to turn the push rod and additional 1/2 turn so that the piston rotates freely. |
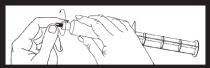 | Prior to using the syringe, twist off tip cap and discard. The area under the tip cap is sterile, caution should now be used when handling. Syringe is now ready for needle or infusion tubing attachment.
|
125 mL Syringe:
| Assembly and Inspection
NOTE: Exterior of syringe is not sterile. |
| Contents of syringe and area under tip cap and piston ribs are sterile and should be treated accordingly. |
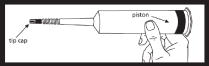 |
Remove syringe from carton and inspect the area around the tip cap and outside of piston for signs of leakage. Do not use if leakage is observed. Load syringe into power injector.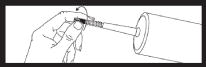 |
| To remove tip cap from syringe, push in and twist off, then discard. The area under the cap is sterile. Caution should now be used when handling. Luer Locknut Detail: 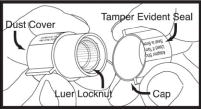 |
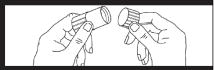 |
Next remove cap from luer locknut dust cover by twisting to break tamper evident seal. Discard cap.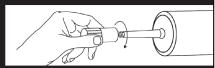 Attach luer locknut to syringe by holding dust cover and screwing to the stop. Attach luer locknut to syringe by holding dust cover and screwing to the stop.Remove and discard dust cover when ready to attach sterile connector tubing. |
| Sodium Chloride (Sodium Chloride) | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
Revised: 10/2006