abilify (aripiprazole) tablet
abilify discmelt (aripiprazole) tablet, orally disintegrating
abilify (aripiprazole) solution
abilify (aripiprazole) injection, solution
[Otsuka Pharmaceutical Co, Ltd]
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks) in these patients revealed a risk of death in the drug-treated patients of between 1.6 to 1.7 times that seen in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis.
DESCRIPTION
Aripiprazole is a psychotropic drug that is available as ABILIFY® (aripiprazole) tablets, ABILIFY® DISCMELT™ (aripiprazole) orally disintegrating tablets, ABILIFY® (aripiprazole) oral solution, and ABILIFY® (aripiprazole) injection, a solution for intramuscular injection. Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril. The empirical formula is C23H27Cl2N3O2 and its molecular weight is 448.39. The chemical structure is:
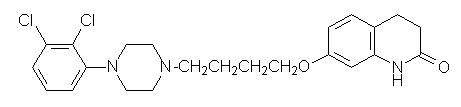
ABILIFY tablets are available in 2-mg, 5-mg, 10-mg, 15-mg, 20-mg, and 30-mg strengths. Inactive ingredients include cornstarch, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake.
ABILIFY DISCMELT orally disintegrating tablets are available in 10-mg and 15-mg strengths. Inactive ingredients include acesulfame potassium, aspartame, calcium silicate, croscarmellose sodium, crospovidone, crème de vanilla (natural and artificial flavors), magnesium stearate, microcrystalline cellulose, silicon dioxide, tartaric acid, and xylitol. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake.
ABILIFY is also available as a 1-mg/mL oral solution. The inactive ingredients for this solution include disodium edetate, fructose, glycerin, dl-lactic acid, methylparaben, propylene glycol, propylparaben, sodium hydroxide, sucrose, and purified water. The oral solution is flavored with natural orange cream and other natural flavors.
ABILIFY Injection is available in single-dose vials as a ready-to-use, 9.75 mg/1.3 mL (7.5 mg/mL), clear, colorless, sterile, aqueous solution for intramuscular use only. Inactive ingredients for this solution include 150 mg/mL of sulfobutyletherβ-cyclodextrin (SBECD), tartaric acid, sodium hydroxide, and water for injection.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Aripiprazole exhibits high affinity for dopamine D2 and D3, serotonin 5-HT1A and 5-HT2A receptors (Ki values of 0.34, 0.8, 1.7, and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic and histamine H1 receptors (Ki values of 44, 15, 39, 57, and 61 nM, respectively), and moderate affinity for the serotonin reuptake site (Ki=98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors (IC50>1000 nM). Aripiprazole functions as a partial agonist at the dopamine D2 and the serotonin 5-HT1A receptors, and as an antagonist at serotonin 5-HT2A receptor.
The mechanism of action of aripiprazole, as with other drugs having efficacy in schizophrenia, bipolar disorder, and agitation associated with schizophrenia or bipolar disorder, is unknown. However, it has been proposed that the efficacy of aripiprazole is mediated through a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. Actions at receptors other than D2, 5-HT1A, and 5-HT2A may explain some of the other clinical effects of aripiprazole, eg, the orthostatic hypotension observed with aripiprazole may be explained by its antagonist activity at adrenergic alpha1 receptors.
Pharmacokinetics
ABILIFY activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents 40% of the parent drug exposure in plasma. The mean elimination half-lives are about 75 hours and 94 hours for aripiprazole and dehydro-aripiprazole, respectively. Steady-state concentrations are attained within 14 days of dosing for both active moieties. Aripiprazole accumulation is predictable from single-dose pharmacokinetics. At steady state, the pharmacokinetics of aripiprazole are dose-proportional. Elimination of aripiprazole is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP3A4.
Pharmacokinetic studies showed that ABILIFY DISCMELT orally disintegrating tablets are bioequivalent to ABILIFY tablets.
ORAL ADMINISTRATION
Absorption
Tablet
Aripiprazole is well absorbed after administration of the tablet, with peak plasma concentrations occurring within 3 to 5 hours; the absolute oral bioavailability of the tablet formulation is 87%. ABILIFY can be administered with or without food. Administration of a 15-mg ABILIFY tablet with a standard high-fat meal did not significantly affect the Cmax or AUC of aripiprazole or its active metabolite, dehydro-aripiprazole, but delayed Tmax by 3 hours for aripiprazole and 12 hours for dehydro-aripiprazole.
Oral Solution
Aripiprazole is well absorbed when administered orally as the solution. At equivalent doses, the plasma concentrations of aripiprazole from the solution were higher than that from the tablet formulation. In a relative bioavailability study comparing the pharmacokinetics of 30 mg aripiprazole as the oral solution to 30-mg aripiprazole tablets in healthy subjects, the solution to tablet ratios of geometric mean Cmax and AUC values were 122% and 114%, respectively (see DOSAGE AND ADMINISTRATION). The single-dose pharmacokinetics of aripiprazole were linear and dose-proportional between the doses of 5 to 30 mg.
Distribution
The steady-state volume of distribution of aripiprazole following intravenous administration is high (404 L or 4.9 L/kg), indicating extensive extravascular distribution. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin. In healthy human volunteers administered 0.5 to 30 mg/day aripiprazole for 14 days, there was dose-dependent D2 receptor occupancy indicating brain penetration of aripiprazole in humans.
Metabolism and Elimination
Aripiprazole is metabolized primarily by three biotransformation pathways: dehydrogenation, hydroxylation, and N-dealkylation. Based on in vitro studies, CYP3A4 and CYP2D6 enzymes are responsible for dehydrogenation and hydroxylation of aripiprazole, and N-dealkylation is catalyzed by CYP3A4. Aripiprazole is the predominant drug moiety in the systemic circulation. At steady state, dehydro-aripiprazole, the active metabolite, represents about 40% of aripiprazole AUC in plasma.
Approximately 8% of Caucasians lack the capacity to metabolize CYP2D6 substrates and are classified as poor metabolizers (PM), whereas the rest are extensive metabolizers (EM). PMs have about an 80% increase in aripiprazole exposure and about a 30% decrease in exposure to the active metabolite compared to EMs, resulting in about a 60% higher exposure to the total active moieties from a given dose of aripiprazole compared to EMs. Coadministration of ABILIFY with known inhibitors of CYP2D6, like quinidine in EMs, results in a 112% increase in aripiprazole plasma exposure, and dosing adjustment is needed (see PRECAUTIONS: Drug-Drug Interactions). The mean elimination half-lives are about 75 hours and 146 hours for aripiprazole in EMs and PMs, respectively. Aripiprazole does not inhibit or induce the CYP2D6 pathway.
Following a single oral dose of [14C]-labeled aripiprazole, approximately 25% and 55% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 1% of unchanged aripiprazole was excreted in the urine and approximately 18% of the oral dose was recovered unchanged in the feces.
INTRAMUSCULAR ADMINISTRATION
In two pharmacokinetic studies of aripiprazole injection administered intramuscularly to healthy subjects, the median times to the peak plasma concentrations were at 1 and 3 hours. A 5-mg intramuscular injection of aripiprazole had an absolute bioavailability of 100%. The geometric mean maximum concentration achieved after an intramuscular dose was on average 19% higher than the Cmax of the oral tablet. While the systemic exposure over 24 hours was generally similar between aripiprazole injection given intramuscularly and after oral tablet administration, the aripiprazole AUC in the first 2 hours after an intramuscular injection was 90% greater than the AUC after the same dose as a tablet. In stable patients with schizophrenia or schizoaffective disorder, the pharmacokinetics of aripiprazole after intramuscular administration were linear over a dose range of 1 to 45 mg. Although the metabolism of aripiprazole injection was not systematically evaluated, the intramuscular route of administration would not be expected to alter the metabolic pathways.
Special Populations
In general, no dosage adjustment for ABILIFY is required on the basis of a patient’s age, gender, race, smoking status, hepatic function, or renal function (see DOSAGE AND ADMINISTRATION: Dosage in Special Populations). The pharmacokinetics of aripiprazole in special populations are described below.
Hepatic Impairment
In a single-dose study (15 mg of aripiprazole) in subjects with varying degrees of liver cirrhosis (Child-Pugh Classes A, B, and C), the AUC of aripiprazole, compared to healthy subjects, increased 31% in mild HI, increased 8% in moderate HI, and decreased 20% in severe HI. None of these differences would require dose adjustment.
Renal Impairment
In patients with severe renal impairment (creatinine clearance <30 mL/min), Cmax of aripiprazole (given in a single dose of 15 mg) and dehydro-aripiprazole increased by 36% and 53%, respectively, but AUC was 15% lower for aripiprazole and 7% higher for dehydro-aripiprazole. Renal excretion of both unchanged aripiprazole and dehydro-aripiprazole is less than 1% of the dose. No dosage adjustment is required in subjects with renal impairment.
Elderly
In formal single-dose pharmacokinetic studies (with aripiprazole given in a single dose of 15 mg), aripiprazole clearance was 20% lower in elderly (≥65 years) subjects compared to younger adult subjects (18 to 64 years). There was no detectable age effect, however, in the population pharmacokinetic analysis in schizophrenia patients. Also, the pharmacokinetics of aripiprazole after multiple doses in elderly patients appeared similar to that observed in young, healthy subjects. No dosage adjustment is recommended for elderly patients (see Boxed WARNING, WARNINGS: Increased Mortality in Elderly Patients with Dementia-Related Psychosis, and PRECAUTIONS: Geriatric Use).
Gender
Cmax and AUC of aripiprazole and its active metabolite, dehydro-aripiprazole, are 30 to 40% higher in women than in men, and correspondingly, the apparent oral clearance of aripiprazole is lower in women. These differences, however, are largely explained by differences in body weight (25%) between men and women. No dosage adjustment is recommended based on gender.
Race
Although no specific pharmacokinetic study was conducted to investigate the effects of race on the disposition of aripiprazole, population pharmacokinetic evaluation revealed no evidence of clinically significant race-related differences in the pharmacokinetics of aripiprazole. No dosage adjustment is recommended based on race.
Smoking
Based on studies utilizing human liver enzymes in vitro, aripiprazole is not a substrate for CYP1A2 and also does not undergo direct glucuronidation. Smoking should, therefore, not have an effect on the pharmacokinetics of aripiprazole. Consistent with these in vitro results, population pharmacokinetic evaluation did not reveal any significant pharmacokinetic differences between smokers and nonsmokers. No dosage adjustment is recommended based on smoking status.
Drug-Drug Interactions
Potential for Other Drugs to Affect ABILIFY
Aripiprazole is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes. Aripiprazole also does not undergo direct glucuronidation. This suggests that an interaction of aripiprazole with inhibitors or inducers of these enzymes, or other factors, like smoking, is unlikely.
Both CYP3A4 and CYP2D6 are responsible for aripiprazole metabolism. Agents that induce CYP3A4 (eg, carbamazepine) could cause an increase in aripiprazole clearance and lower blood levels. Inhibitors of CYP3A4 (eg, ketoconazole) or CYP2D6 (eg, quinidine, fluoxetine, or paroxetine) can inhibit aripiprazole elimination and cause increased blood levels.
Valproate: When valproate (500-1500 mg/day) and aripiprazole (30 mg/day) were coadministered at steady state, the Cmax and AUC of aripiprazole were decreased by 25%. No dosage adjustment of aripiprazole is required when administered concomitantly with valproate.
Lithium: A pharmacokinetic interaction of aripiprazole with lithium is unlikely because lithium is not bound to plasma proteins, is not metabolized, and is almost entirely excreted unchanged in urine. Coadministration of therapeutic doses of lithium (1200-1800 mg/day) for 21 days with aripiprazole (30 mg/day) did not result in clinically significant changes in the pharmacokinetics of aripiprazole or its active metabolite, dehydro-aripiprazole (Cmax and AUC increased by less than 20%). No dosage adjustment of aripiprazole is required when administered concomitantly with lithium.
Potential for ABILIFY to Affect Other Drugs
Aripiprazole is unlikely to cause clinically important pharmacokinetic interactions with drugs metabolized by cytochrome P450 enzymes. In in vivo studies, 10- to 30-mg/day doses of aripiprazole had no significant effect on metabolism by CYP2D6 (dextromethorphan), CYP2C9 (warfarin), CYP2C19 (omeprazole, warfarin), and CYP3A4 (dextromethorphan) substrates. Additionally, aripiprazole and dehydro-aripiprazole did not show potential for altering CYP1A2-mediated metabolism in vitro (see PRECAUTIONS: Drug-Drug Interactions).
Aripiprazole had no clinically important interactions with the following drugs:
Famotidine: Coadministration of aripiprazole (given in a single dose of 15 mg) with a 40-mg single dose of the H2 antagonist famotidine, a potent gastric acid blocker, decreased the solubility of aripiprazole and, hence, its rate of absorption, reducing by 37% and 21% the Cmax of aripiprazole and dehydro-aripiprazole, respectively, and by 13% and 15%, respectively, the extent of absorption (AUC). No dosage adjustment of aripiprazole is required when administered concomitantly with famotidine.
Valproate: When aripiprazole (30 mg/day) and valproate (1000 mg/day) were coadministered at steady state, there were no clinically significant changes in the Cmax or AUC of valproate. No dosage adjustment of valproate is required when administered concomitantly with aripiprazole.
Lithium: Coadministration of aripiprazole (30 mg/day) with lithium (900 mg/day) did not result in clinically significant changes in the pharmacokinetics of lithium. No dosage adjustment of lithium is required when administered concomitantly with aripiprazole.
Dextromethorphan: Aripiprazole at doses of 10 to 30 mg per day for 14 days had no effect on dextromethorphan’s O-dealkylation to its major metabolite, dextrorphan, a pathway known to be dependent on CYP2D6 activity. Aripiprazole also had no effect on dextromethorphan’s N-demethylation to its metabolite 3-methyoxymorphan, a pathway known to be dependent on CYP3A4 activity. No dosage adjustment of dextromethorphan is required when administered concomitantly with aripiprazole.
Warfarin: Aripiprazole 10 mg per day for 14 days had no effect on the pharmacokinetics of R- and S-warfarin or on the pharmacodynamic end point of International Normalized Ratio, indicating the lack of a clinically relevant effect of aripiprazole on CYP2C9 and CYP2C19 metabolism or the binding of highly protein-bound warfarin. No dosage adjustment of warfarin is required when administered concomitantly with aripiprazole.
Omeprazole: Aripiprazole 10 mg per day for 15 days had no effect on the pharmacokinetics of a single 20-mg dose of omeprazole, a CYP2C19 substrate, in healthy subjects. No dosage adjustment of omeprazole is required when administered concomitantly with aripiprazole.
Lorazepam: Coadministration of lorazepam injection (2 mg) and aripiprazole injection (15 mg) to healthy subjects (n=40: 35 males and 5 females; ages 19-45 years old) did not result in clinically important changes in the pharmacokinetics of either drug. No dosage adjustment of aripiprazole is required when administered concomitantly with lorazepam. However, the intensity of sedation was greater with the combination as compared to that observed with aripiprazole alone and the orthostatic hypotension observed was greater with the combination as compared to that observed with lorazepam alone (see PRECAUTIONS: General).
Clinical Studies
Schizophrenia
The efficacy of ABILIFY (aripiprazole) in the treatment of schizophrenia was evaluated in five short-term (4- and 6-week), placebo-controlled trials of acutely relapsed inpatients who predominantly met DSM-III/IV criteria for schizophrenia. Four of the five trials were able to distinguish aripiprazole from placebo, but one study, the smallest, did not. Three of these studies also included an active control group consisting of either risperidone (one trial) or haloperidol (two trials), but they were not designed to allow for a comparison of ABILIFY and the active comparators.
In the four positive trials for ABILIFY, four primary measures were used for assessing psychiatric signs and symptoms. The Positive and Negative Syndrome Scale (PANSS) is a multi-item inventory of general psychopathology used to evaluate the effects of drug treatment in schizophrenia. The PANSS positive subscale is a subset of items in the PANSS that rates seven positive symptoms of schizophrenia (delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness/persecution, and hostility). The PANSS negative subscale is a subset of items in the PANSS that rates seven negative symptoms of schizophrenia (blunted affect, emotional withdrawal, poor rapport, passive apathetic withdrawal, difficulty in abstract thinking, lack of spontaneity/flow of conversation, and stereotyped thinking). The Clinical Global Impression (CGI) assessment reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
In a 4-week trial (n=414) comparing two fixed doses of ABILIFY (15 or 30 mg/day) and haloperidol (10 mg/day) to placebo, both doses of ABILIFY were superior to placebo in the PANSS total score, PANSS positive subscale, and CGI-severity score. In addition, the 15-mg dose was superior to placebo in the PANSS negative subscale.
In a 4-week trial (n=404) comparing two fixed doses of ABILIFY (20 or 30 mg/day) and risperidone (6 mg/day) to placebo, both doses of ABILIFY were superior to placebo in the PANSS total score, PANSS positive subscale, PANSS negative subscale, and CGI-severity score.
In a 6-week trial (n=420) comparing three fixed doses of ABILIFY (10, 15, or 20 mg/day) to placebo, all three doses of ABILIFY were superior to placebo in the PANSS total score, PANSS positive subscale, and the PANSS negative subscale.
In a 6-week trial (n=367) comparing three fixed doses of ABILIFY (2, 5, or 10 mg/day) to placebo, the 10-mg dose of ABILIFY was superior to placebo in the PANSS total score, the primary outcome measure of the study. The 2-mg and 5-mg doses did not demonstrate superiority to placebo on the primary outcome measure.
In a fifth study, a 4-week trial (n=103) comparing ABILIFY in a range of 5 to 30 mg/day or haloperidol 5 to 20 mg/day to placebo, haloperidol was superior to placebo, in the Brief Psychiatric Rating Scale (BPRS), a multi-item inventory of general psychopathology traditionally used to evaluate the effects of drug treatment in psychosis, and in a responder analysis based on the CGI-severity score, the primary outcomes for that trial. ABILIFY was only significantly different compared to placebo in a responder analysis based on the CGI-severity score.
Thus, the efficacy of 10-mg, 15-mg, 20-mg, and 30-mg daily doses was established in two studies for each dose. Among these doses, there was no evidence that the higher dose groups offered any advantage over the lowest dose group of these studies.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender, or race.
A longer-term trial enrolled 310 inpatients or outpatients meeting DSM-IV criteria for schizophrenia who were, by history, symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from their antipsychotic medications and randomized to ABILIFY 15 mg or placebo for up to 26 weeks of observation for relapse. Relapse during the double-blind phase was defined as CGI-Improvement score of ≥5 (minimally worse), scores≥5 (moderately severe) on the hostility or uncooperativeness items of the PANSS, or ≥20% increase in the PANSS total score. Patients receiving ABILIFY 15 mg experienced a significantly longer time to relapse over the subsequent 26 weeks compared to those receiving placebo.
Bipolar Disorder
The efficacy of ABILIFY in the treatment of acute manic episodes was established in two 3-week, placebo-controlled trials in hospitalized patients who met the DSM-IV criteria for Bipolar I Disorder with manic or mixed episodes (in one trial, 21% of placebo and 42% of ABILIFY-treated patients had data beyond two weeks). These trials included patients with or without psychotic features and with or without a rapid-cycling course.
The primary instrument used for assessing manic symptoms was the Young Mania Rating Scale (Y-MRS), an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology (irritability, disruptive/aggressive behavior, sleep, elevated mood, speech, increased activity, sexual interest, language/thought disorder, thought content, appearance, and insight) in a range from 0 (no manic features) to 60 (maximum score). A key secondary instrument included the Clinical Global Impression - Bipolar (CGI-BP) scale.
In the two positive, 3-week, placebo-controlled trials (n=268; n=248) which evaluated ABILIFY 15 or 30 mg/day, once daily (with a starting dose of 30 mg/day), ABILIFY was superior to placebo in the reduction of Y-MRS total score and CGI-BP Severity of Illness score (mania).
A trial was conducted in patients meeting DSM-IV criteria for Bipolar I Disorder with a recent manic or mixed episode who had been stabilized on open-label ABILIFY and who had maintained a clinical response for at least 6 weeks. The first phase of this trial was an open-label stabilization period in which inpatients and outpatients were clinically stabilized and then maintained on open-label ABILIFY (15 or 30 mg/day, with a starting dose of 30 mg/day) for at least 6 consecutive weeks. One hundred sixty-one outpatients were then randomized in a double-blind fashion, to either the same dose of ABILIFY they were on at the end of the stabilization and maintenance period or placebo and were then monitored for manic or depressive relapse. During the randomization phase, ABILIFY was superior to placebo on time to the number of combined affective relapses (manic plus depressive), the primary outcome measure for this study. The majority of these relapses were due to manic rather than depressive symptoms. There is insufficient data to know whether ABILIFY is effective in delaying the time to occurrence of depression in patients with Bipolar I Disorder.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender; however, there were insufficient numbers of patients in each of the ethnic groups to adequately assess inter-group differences.
Agitation Associated with Schizophrenia or Bipolar Mania
The efficacy of intramuscular aripiprazole for injection for the treatment of agitation was established in three short-term (24-hour), placebo-controlled trials in agitated inpatients from two diagnostic groups: schizophrenia and Bipolar I Disorder (manic or mixed episodes, with or without psychotic features). Each of the trials included a single active comparator treatment arm of either haloperidol injection (schizophrenia studies) or lorazepam injection (bipolar mania study). Patients could receive up to three injections during the 24-hour treatment periods; however, patients could not receive the second injection until after the initial 2-hour period when the primary efficacy measure was assessed. Patients enrolled in the trials needed to be: (1) judged by the clinical investigators as clinically agitated and clinically appropriate candidates for treatment with intramuscular medication, and (2) exhibiting a level of agitation that met or exceeded a threshold score of ≥15 on the five items comprising the Positive and Negative Syndrome Scale (PANSS) Excited Component (ie, poor impulse control, tension, hostility, uncooperativeness, and excitement items) with at least two individual item scores ≥4 using a 1-7 scoring system (1 = absent, 4 = moderate, 7 = extreme). In the studies, the mean baseline PANSS Excited Component score was 19, with scores ranging from 15 to 34 (out of a maximum score of 35), thus suggesting predominantly moderate levels of agitation with some patients experiencing mild or severe levels of agitation. The primary efficacy measure used for assessing agitation signs and symptoms in these trials was the change from baseline in the PANSS Excited Component at 2 hours post-injection. A key secondary measure was the Clinical Global Impression of Improvement (CGI-I) scale. The results of the trials follow:
- (1) In a placebo-controlled trial in agitated inpatients predominantly meeting DSM-IV criteria for schizophrenia (n=350), four fixed aripiprazole injection doses of 1 mg, 5.25 mg, 9.75 mg, and 15 mg were evaluated. At 2 hours post-injection, the 5.25-mg, 9.75-mg, and 15-mg doses were statistically superior to placebo in the PANSS Excited Component and on the CGI-I scale.
- (2) In a second placebo-controlled trial in agitated inpatients predominantly meeting DSM-IV criteria for schizophrenia (n=445), one fixed aripiprazole injection dose of 9.75 mg was evaluated. At 2 hours post-injection, aripiprazole for injection was statistically superior to placebo in the PANSS Excited Component and on the CGI-I scale.
- (3) In a placebo-controlled trial in agitated inpatients meeting DSM-IV criteria for Bipolar I Disorder (manic or mixed) (n=291), two fixed aripiprazole injection doses of 9.75 mg and 15 mg were evaluated. At 2 hours post-injection, both doses were statistically superior to placebo in the PANSS Excited Component.
Examination of population subsets (age, race, and gender) did not reveal any differential responsiveness on the basis of these subgroupings.
INDICATIONS AND USAGE
Schizophrenia
ABILIFY is indicated for the treatment of schizophrenia. The efficacy of ABILIFY in the treatment of schizophrenia was established in short-term (4- and 6-week) controlled trials of schizophrenic inpatients (see CLINICAL PHARMACOLOGY: Clinical Studies).
The efficacy of ABILIFY in maintaining stability in patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer, were discontinued from those other medications, and were then administered ABILIFY 15 mg/day and observed for relapse during a period of up to 26 weeks was demonstrated in a placebo-controlled trial (see CLINICAL PHARMACOLOGY: Clinical Studies). The physician who elects to use ABILIFY for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
Bipolar Disorder
ABILIFY is indicated for the treatment of acute manic and mixed episodes associated with Bipolar Disorder.
The efficacy of ABILIFY was established in two placebo-controlled trials (3 week) of inpatients with DSM-IV criteria for Bipolar I Disorder who were experiencing an acute manic or mixed episode with or without psychotic features (see CLINICAL PHARMACOLOGY: Clinical Studies).
The efficacy of ABILIFY in maintaining efficacy in patients with Bipolar I Disorder with a recent manic or mixed episode who had been stabilized and then maintained for at least 6 weeks, was demonstrated in a double-blind, placebo-controlled trial. Prior to entering the double-blind, randomization phase of this trial, patients were clinically stabilized and maintained their stability for 6 consecutive weeks on ABILIFY. Following this 6-week maintenance phase, patients were randomized to either placebo or ABILIFY and monitored for relapse (see CLINICAL PHARMACOLOGY: Clinical Studies). Physicians who elect to use ABILIFY for extended periods, that is, longer than 6 weeks, should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see DOSAGE AND ADMINISTRATION).
Agitation Associated with Schizophrenia or Bipolar Mania
ABILIFY Injection is indicated for the treatment of agitation associated with schizophrenia or bipolar disorder, manic or mixed. "Psychomotor agitation" is defined in DSM-IV as "excessive motor activity associated with a feeling of inner tension." Patients experiencing agitation often manifest behaviors that interfere with their diagnosis and care (eg, threatening behaviors, escalating or urgently distressing behavior, or self-exhausting behavior), leading clinicians to the use of intramuscular antipsychotic medications to achieve immediate control of the agitation.
The efficacy of ABILIFY Injection for the treatment of agitation associated with schizophrenia or Bipolar I Disorder was established in three short-term (24-hour), placebo-controlled trials in agitated inpatients with schizophrenia or Bipolar I Disorder (manic or mixed episodes) (see CLINICAL PHARMACOLOGY: Clinical Studies).
CONTRAINDICATIONS
ABILIFY is contraindicated in patients with a known hypersensitivity to the product.
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis (see Boxed WARNING).
Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including aripiprazole. Rare cases of NMS occurred during aripiprazole treatment in the worldwide clinical database. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to exclude cases where the clinical presentation includes both serious medical illness (eg, pneumonia, systemic infection, etc) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and, thereby, may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, ABILIFY should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on ABILIFY, drug discontinuation should be considered. However, some patients may require treatment with ABILIFY despite the presence of the syndrome.
Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled clinical studies (two flexible dose and one fixed dose study) of dementia-related psychosis, there was an increased incidence of cerebrovascular adverse events (eg, stroke, transient ischemic attack), including fatalities, in aripiprazole-treated patients (mean age: 84 years; range: 78-88 years). In the fixed-dose study, there was a statistically significant dose response relationship for cerebrovascular adverse events in patients treated with aripiprazole. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis. (See also Boxed WARNING, WARNINGS: Increased Mortality in Elderly Patients with Dementia-Related Psychosis, and PRECAUTIONS: Use in Patients with Concomitant Illness: Safety Experience in Elderly Patients with Psychosis Associated with Alzheimer’s Disease.)
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. There have been few reports of hyperglycemia in patients treated with ABILIFY. Although fewer patients have been treated with ABILIFY, it is not known if this more limited experience is the sole reason for the paucity of such reports. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies which did not include ABILIFY suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics included in these studies. Because ABILIFY was not marketed at the time these studies were performed, it is not known if ABILIFY is associated with this increased risk. Precise risk estimates for hyperglycemia-related adverse events in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (eg, obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
PRECAUTIONS
General
Orthostatic Hypotension
Aripiprazole may be associated with orthostatic hypotension, perhaps due to its α1-adrenergic receptor antagonism. The incidence of orthostatic hypotension-associated events from five short-term, placebo-controlled trials in schizophrenia (n=926) on oral ABILIFY included: orthostatic hypotension (placebo 1%, aripiprazole 1.9%), postural dizziness (placebo 0.7%, aripiprazole 0.8%), and syncope (placebo 1%, aripiprazole 0.6%). The incidence of orthostatic hypotension-associated events from short-term, placebo-controlled trials in bipolar mania (n=597) on oral ABILIFY included: orthostatic hypotension (placebo 0%, aripiprazole 0.7%), postural dizziness (placebo 0.2%, aripiprazole 0.5%), and syncope (placebo 0.7%, aripiprazole 0.3%). The incidence of orthostatic hypotension-associated events from short-term, placebo-controlled trials in agitation associated with schizophrenia or bipolar mania (n=501) on ABILIFY Injection included: orthostatic hypotension (placebo 0%, aripiprazole 0.6%), postural dizziness (placebo 0.5%, aripiprazole 0.2%), and syncope (placebo 0%, aripiprazole 0.4%).
The incidence of a significant orthostatic change in blood pressure (defined as a decrease of at least 30 mmHg in systolic blood pressure when changing from a supine to standing position) for aripiprazole was not statistically different from placebo (in schizophrenia: 14% among oral aripiprazole-treated patients and 12% among placebo-treated patients, in bipolar mania: 3% among oral aripiprazole-treated patients and 2% among placebo-treated patients, and in patients with agitation associated with schizophrenia or bipolar mania: 4% among aripiprazole injection-treated patients and 4% among placebo-treated patients).
Aripiprazole should be used with caution in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease, or conditions which would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications).
If parenteral benzodiazepine therapy is deemed necessary in addition to aripiprazole injection treatment, patients should be monitored for excessive sedation and for orthostatic hypotension (see CLINICAL PHARMACOLOGY: Drug-Drug Interactions).
Seizure/Convulsion
Seizures/convulsions occurred in 0.1% (1/926) of oral aripiprazole-treated patients with schizophrenia in short-term, placebo-controlled trials. In short-term, placebo-controlled clinical trials of patients with bipolar mania, 0.3% (2/597) of oral aripiprazole-treated patients and 0.2% (1/436) of placebo-treated patients experienced seizures. In short-term, placebo-controlled clinical trials of patients with agitation associated with schizophrenia or bipolar mania, 0.2% (1/501) of aripiprazole injection-treated patients and 0% (0/220) of placebo-treated patients experienced seizures.
As with other antipsychotic drugs, aripiprazole should be used cautiously in patients with a history of seizures or with conditions that lower the seizure threshold, eg, Alzheimer’s dementia. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
Potential for Cognitive and Motor Impairment
ABILIFY, like other antipsychotics, may have the potential to impair judgment, thinking, or motor skills. For example, in short-term, placebo-controlled trials of schizophrenia, somnolence (including sedation) was reported in 10% of patients on oral ABILIFY compared to 8% of patients on placebo. Somnolence (including sedation) led to discontinuation in 0.1% (1/926) of patients with schizophrenia on oral ABILIFY in short-term, placebo-controlled trials. In short-term, placebo-controlled trials of bipolar mania, somnolence (including sedation) was reported in 14% of patients on oral ABILIFY compared to 7% of patients on placebo, but did not lead to discontinuation of any patients with bipolar mania. In short-term, placebo-controlled trials of patients with agitation associated with schizophrenia or bipolar mania, somnolence (including sedation) was reported in 9% of patients on ABILIFY Injection compared to 6% of patients on placebo. Somnolence (including sedation) did not lead to discontinuation of any patients with agitation associated with schizophrenia or bipolar mania.
Despite the relatively modest increased incidence of somnolence compared to placebo, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with ABILIFY does not affect them adversely.
Body Temperature Regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing aripiprazole for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, eg, exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use, including ABILIFY. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer’s dementia. Aripiprazole and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia (see PRECAUTIONS: Use in Patients with Concomitant Illness).
Suicide
The possibility of a suicide attempt is inherent in psychotic illnesses and bipolar disorder, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for ABILIFY should be written for the smallest quantity consistent with good patient management in order to reduce the risk of overdose.
Use in Patients with Concomitant Illness
Clinical experience with ABILIFY in patients with certain concomitant systemic illnesses (see CLINICAL PHARMACOLOGY: Special Populations: Renal Impairment and Hepatic Impairment) is limited.
ABILIFY has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were excluded from premarketing clinical studies.
Safety Experience in Elderly Patients with Psychosis Associated with Alzheimer’s Disease: In three, 10-week, placebo-controlled studies of aripiprazole in elderly patients with psychosis associated with Alzheimer’s disease (n=938; mean age: 82.4 years; range: 56-99 years), the treatment-emergent adverse events that were reported at an incidence of ≥3% and aripiprazole incidence at least twice that for placebo were lethargy [placebo 2%, aripiprazole 5%], somnolence (including sedation) [placebo 3%, aripiprazole 8%], and incontinence (primarily, urinary incontinence) [placebo 1%, aripiprazole 5%], excessive salivation [placebo 0%, aripiprazole 4%], and lightheadedness [placebo 1%, aripiprazole 4%].
The safety and efficacy of ABILIFY in the treatment of patients with psychosis associated with dementia have not been established. If the prescriber elects to treat such patients with ABILIFY, vigilance should be exercised, particularly for the emergence of difficulty swallowing or excessive somnolence, which could predispose to accidental injury or aspiration. (See also Boxed WARNING, WARNINGS: Increased Mortality in Elderly Patients with Dementia-Related Psychosis, and Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia-Related Psychosis.)
Information for Patients
Physicians are advised to discuss the following issues with patients for whom they prescribe ABILIFY:
Interference with Cognitive and Motor Performance
Because aripiprazole may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that aripiprazole therapy does not affect them adversely.
Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy with ABILIFY.
Nursing
Patients should be advised not to breast-feed an infant if they are taking ABILIFY.
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
Alcohol
Patients should be advised to avoid alcohol while taking ABILIFY.
Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration.
Sugar Content
Patients should be advised that each mL of ABILIFY oral solution contains 400 mg of sucrose and 200 mg of fructose.
Phenylketonurics
Phenylalanine is a component of aspartame. Each ABILIFY DISCMELT orally disintegrating tablet contains the following amounts: 10 mg - 1.12 mg phenylalanine and 15 mg - 1.68 mg phenylalanine.
Drug-Drug Interactions
Given the primary CNS effects of aripiprazole, caution should be used when ABILIFY is taken in combination with other centrally acting drugs and alcohol. Due to its α1-adrenergic receptor antagonism, aripiprazole has the potential to enhance the effect of certain antihypertensive agents.
Potential for Other Drugs to Affect ABILIFY
Aripiprazole is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes. Aripiprazole also does not undergo direct glucuronidation. This suggests that an interaction of aripiprazole with inhibitors or inducers of these enzymes, or other factors, like smoking, is unlikely.
Both CYP3A4 and CYP2D6 are responsible for aripiprazole metabolism. Agents that induce CYP3A4 (eg, carbamazepine) could cause an increase in aripiprazole clearance and lower blood levels. Inhibitors of CYP3A4 (eg, ketoconazole) or CYP2D6 (eg, quinidine, fluoxetine, or paroxetine) can inhibit aripiprazole elimination and cause increased blood levels.
Ketoconazole: Coadministration of ketoconazole (200 mg/day for 14 days) with a 15-mg single dose of aripiprazole increased the AUC of aripiprazole and its active metabolite by 63% and 77%, respectively. The effect of a higher ketoconazole dose (400 mg/day) has not been studied. When concomitant administration of ketoconazole with aripiprazole occurs, aripiprazole dose should be reduced to one-half of its normal dose. Other strong inhibitors of CYP3A4 (itraconazole) would be expected to have similar effects and need similar dose reductions; weaker inhibitors (erythromycin, grapefruit juice) have not been studied. When the CYP3A4 inhibitor is withdrawn from the combination therapy, aripiprazole dose should then be increased.
Quinidine: Coadministration of a 10-mg single dose of aripiprazole with quinidine (166 mg/day for 13 days), a potent inhibitor of CYP2D6, increased the AUC of aripiprazole by 112% but decreased the AUC of its active metabolite, dehydro-aripiprazole, by 35%. Aripiprazole dose should be reduced to one-half of its normal dose when concomitant administration of quinidine with aripiprazole occurs. Other significant inhibitors of CYP2D6, such as fluoxetine or paroxetine, would be expected to have similar effects and, therefore, should be accompanied by similar dose reductions. When the CYP2D6 inhibitor is withdrawn from the combination therapy, aripiprazole dose should then be increased.
Carbamazepine: Coadministration of carbamazepine (200 mg BID), a potent CYP3A4 inducer, with aripiprazole (30 mg QD) resulted in an approximate 70% decrease in Cmax and AUC values of both aripiprazole and its active metabolite, dehydro-aripiprazole. When carbamazepine is added to aripiprazole therapy, aripiprazole dose should be doubled. Additional dose increases should be based on clinical evaluation. When carbamazepine is withdrawn from the combination therapy, aripiprazole dose should then be reduced.
No clinically significant effect of famotidine, valproate, or lithium was seen on the pharmacokinetics of aripiprazole (see CLINICAL PHARMACOLOGY: Drug-Drug Interactions).
Potential for ABILIFY to Affect Other Drugs
Aripiprazole is unlikely to cause clinically important pharmacokinetic interactions with drugs metabolized by cytochrome P450 enzymes. In in vivo studies, 10- to 30-mg/day doses of aripiprazole had no significant effect on metabolism by CYP2D6 (dextromethorphan), CYP2C9 (warfarin), CYP2C19 (omeprazole, warfarin), and CYP3A4 (dextromethorphan) substrates. Additionally, aripiprazole and dehydro-aripiprazole did not show potential for altering CYP1A2-mediated metabolism in vitro (see CLINICAL PHARMACOLOGY: Drug-Drug Interactions).
Alcohol: There was no significant difference between aripiprazole coadministered with ethanol and placebo coadministered with ethanol on performance of gross motor skills or stimulus response in healthy subjects. As with most psychoactive medications, patients should be advised to avoid alcohol while taking ABILIFY.
No effect of aripiprazole was seen on the pharmacokinetics of lithium or valproate (see CLINICAL PHARMACOLOGY: Drug-Drug Interactions).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies were conducted in ICR mice and in Sprague-Dawley (SD) and F344 rats. Aripiprazole was administered for 2 years in the diet at doses of 1, 3, 10, and 30 mg/kg/day to ICR mice and 1, 3, and 10 mg/kg/day to F344 rats (0.2 to 5 and 0.3 to 3 times the maximum recommended human dose [MRHD] based on mg/m2, respectively). In addition, SD rats were dosed orally for 2 years at 10, 20, 40, and 60 mg/kg/day (3 to 19 times the MRHD based on mg/m2). Aripiprazole did not induce tumors in male mice or rats. In female mice, the incidences of pituitary gland adenomas and mammary gland adenocarcinomas and adenoacanthomas were increased at dietary doses of 3 to 30 mg/kg/day (0.1 to 0.9 times human exposure at MRHD based on AUC and 0.5 to 5 times the MRHD based on mg/m2). In female rats, the incidence of mammary gland fibroadenomas was increased at a dietary dose of 10 mg/kg/day (0.1 times human exposure at MRHD based on AUC and 3 times the MRHD based on mg/m2); and the incidences of adrenocortical carcinomas and combined adrenocortical adenomas/carcinomas were increased at an oral dose of 60 mg/kg/day (14 times human exposure at MRHD based on AUC and 19 times the MRHD based on mg/m2).
Proliferative changes in the pituitary and mammary gland of rodents have been observed following chronic administration of other antipsychotic agents and are considered prolactin-mediated. Serum prolactin was not measured in the aripiprazole carcinogenicity studies. However, increases in serum prolactin levels were observed in female mice in a 13-week dietary study at the doses associated with mammary gland and pituitary tumors. Serum prolactin was not increased in female rats in 4- and 13-week dietary studies at the dose associated with mammary gland tumors. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unknown.
Mutagenesis
The mutagenic potential of aripiprazole was tested in the in vitro bacterial reverse-mutation assay, the in vitro bacterial DNA repair assay, the in vitro forward gene mutation assay in mouse lymphoma cells, the in vitro chromosomal aberration assay in Chinese hamster lung (CHL) cells, the in vivo micronucleus assay in mice, and the unscheduled DNA synthesis assay in rats. Aripiprazole and a metabolite (2,3-DCPP) were clastogenic in the in vitro chromosomal aberration assay in CHL cells with and without metabolic activation. The metabolite, 2,3-DCPP, produced increases in numerical aberrations in the in vitro assay in CHL cells in the absence of metabolic activation. A positive response was obtained in the in vivo micronucleus assay in mice; however, the response was shown to be due to a mechanism not considered relevant to humans.
Impairment of Fertility
Female rats were treated with oral doses of 2, 6, and 20 mg/kg/day (0.6, 2, and 6 times the maximum recommended human dose [MRHD] on a mg/m2 basis) of aripiprazole from 2 weeks prior to mating through day 7 of gestation. Estrus cycle irregularities and increased corpora lutea were seen at all doses, but no impairment of fertility was seen. Increased pre-implantation loss was seen at 6 and 20 mg/kg, and decreased fetal weight was seen at 20 mg/kg.
Male rats were treated with oral doses of 20, 40, and 60 mg/kg/day (6, 13, and 19 times the MRHD on a mg/m2 basis) of aripiprazole from 9 weeks prior to mating through mating. Disturbances in spermatogenesis were seen at 60 mg/kg, and prostate atrophy was seen at 40 and 60 mg/kg, but no impairment of fertility was seen.
Pregnancy
Pregnancy Category C
In animal studies, aripiprazole demonstrated developmental toxicity, including possible teratogenic effects in rats and rabbits.
Pregnant rats were treated with oral doses of 3, 10, and 30 mg/kg/day (1, 3, and 10 times the maximum recommended human dose [MRHD] on a mg/m2 basis) of aripiprazole during the period of organogenesis. Gestation was slightly prolonged at 30 mg/kg. Treatment caused a slight delay in fetal development, as evidenced by decreased fetal weight (30 mg/kg), undescended testes (30 mg/kg), and delayed skeletal ossification (10 and 30 mg/kg). There were no adverse effects on embryofetal or pup survival. Delivered offspring had decreased bodyweights (10 and 30 mg/kg), and increased incidences of hepatodiaphragmatic nodules and diaphragmatic hernia at 30 mg/kg (the other dose groups were not examined for these findings). (A low incidence of diaphragmatic hernia was also seen in the fetuses exposed to 30 mg/kg.) Postnatally, delayed vaginal opening was seen at 10 and 30 mg/kg and impaired reproductive performance (decreased fertility rate, corpora lutea, implants, and live fetuses, and increased post-implantation loss, likely mediated through effects on female offspring) was seen at 30 mg/kg. Some maternal toxicity was seen at 30 mg/kg; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity.
In pregnant rats receiving aripiprazole injection intravenously (3, 9, and 27 mg/kg/day) during the period of organogenesis, decreased fetal weight and delayed skeletal ossification were seen at the highest dose, which also caused some maternal toxicity.
Pregnant rabbits were treated with oral doses of 10, 30, and 100 mg/kg/day (2, 3, and 11 times human exposure at MRHD based on AUC and 6, 19, and 65 times the MRHD based on mg/m2) of aripiprazole during the period of organogenesis. Decreased maternal food consumption and increased abortions were seen at 100 mg/kg. Treatment caused increased fetal mortality (100 mg/kg), decreased fetal weight (30 and 100 mg/kg), increased incidence of a skeletal abnormality (fused sternebrae at 30 and 100 mg/kg) and minor skeletal variations (100 mg/kg).
In pregnant rabbits receiving aripiprazole injection intravenously (3, 10, and 30 mg/kg/day) during the period of organogenesis, the highest dose, which caused pronounced maternal toxicity, resulted in decreased fetal weight, increased fetal abnormalities (primarily skeletal), and decreased fetal skeletal ossification. The fetal no-effect dose was 10 mg/kg, which produced 15 times the human exposure at the MRHD based on AUC, and is 6 times the MRHD based on mg/m2.
In a study in which rats were treated with oral doses of 3, 10, and 30 mg/kg/day (1, 3, and 10 times the MRHD on a mg/m2 basis) of aripiprazole perinatally and postnatally (from day 17 of gestation through day 21 postpartum), slight maternal toxicity and slightly prolonged gestation were seen at 30 mg/kg. An increase in stillbirths, and decreases in pup weight (persisting into adulthood) and survival, were seen at this dose.
In rats receiving aripiprazole injection intravenously (3, 8, and 20 mg/kg/day) from day 6 of gestation through day 20 postpartum, an increase in stillbirths was seen at 8 and 20 mg/kg, and decreases in early postnatal pup weights and survival were seen at 20 mg/kg. These doses produced some maternal toxicity. There were no effects on postnatal behavioral and reproductive development.
There are no adequate and well-controlled studies in pregnant women. It is not known whether aripiprazole can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Aripiprazole should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Labor and Delivery
The effect of aripiprazole on labor and delivery in humans is unknown.
Nursing Mothers
Aripiprazole was excreted in milk of rats during lactation. It is not known whether aripiprazole or its metabolites are excreted in human milk. It is recommended that women receiving aripiprazole should not breast-feed.
Pediatric Use
Safety and effectiveness in pediatric and adolescent patients have not been established.
Geriatric Use
Of the 8456 patients treated with oral aripiprazole in clinical trials, 1000 (12%) were ≥65 years old and 794 (9%) were ≥75 years old. The majority (87%) of the 1000 patients were diagnosed with dementia of the Alzheimer’s type.
Placebo-controlled studies of oral aripiprazole in schizophrenia or bipolar mania did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. There was no effect of age on the pharmacokinetics of a single 15-mg dose of aripiprazole. Aripiprazole clearance was decreased by 20% in elderly subjects (≥65 years) compared to younger adult subjects (18 to 64 years), but there was no detectable effect of age in the population pharmacokinetic analysis in schizophrenia patients.
Of the 749 patients treated with aripiprazole injection in clinical trials, 99 (13%) were ≥65 years old and 78 (10%) were ≥75 years old. Placebo-controlled studies of aripiprazole injection in patients with agitation associated with schizophrenia or bipolar mania did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Studies of elderly patients with psychosis associated with Alzheimer’s disease have suggested that there may be a different tolerability profile in this population compared to younger patients with schizophrenia (see Boxed WARNING, WARNINGS: Increased Mortality in Elderly Patients with Dementia-Related Psychosis; Cerebrovascular Adverse Events, Including Stroke, in Elderly Patients with Dementia -Related Psychosis, and PRECAUTIONS: Use in Patients with Concomitant Illness). The safety and efficacy of ABILIFY in the treatment of patients with psychosis associated with Alzheimer’s disease has not been established. If the prescriber elects to treat such patients with ABILIFY, vigilance should be exercised.
ADVERSE REACTIONS
Aripiprazole has been evaluated for safety in 8456 patients who participated in multiple-dose, clinical trials in schizophrenia, bipolar mania, and dementia of the Alzheimer’s type, and who had approximately 5635 patient-years of exposure to oral aripiprazole and 749 patients with exposure to aripiprazole injection. A total of 2442 patients were treated with oral aripiprazole for at least 180 days and 1667 patients treated with oral aripiprazole had at least 1 year of exposure.
The conditions and duration of treatment with aripiprazole included (in overlapping categories) double-blind, comparative and noncomparative open-label studies, inpatient and outpatient studies, fixed- and flexible-dose studies, and short- and longer-term exposure.
Adverse events during exposure were obtained by collecting volunteered adverse events, as well as results of physical examinations, vital signs, weights, laboratory analyses, and ECG. Adverse experiences were recorded by clinical investigators using terminology of their own choosing. In the tables and tabulations that follow, MedDRA dictionary terminology has been used to classify reported adverse events into a smaller number of standardized event categories, in order to provide a meaningful estimate of the proportion of individuals experiencing adverse events.
The stated frequencies of adverse events represent the proportion of individuals who experienced at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation. There was no attempt to use investigator causality assessments; ie, all reported events are included.
The prescriber should be aware that the figures in the tables and tabulations cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatment, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the adverse event incidence in the population studied.
ORAL ADMINISTRATION
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials of Patients with Schizophrenia
The following findings are based on a pool of five placebo-controlled trials (four 4-week and one 6-week) in which oral aripiprazole was administered in doses ranging from 2 to 30 mg/day.
Adverse Events Associated with Discontinuation of Treatment in Short-Term, Placebo-Controlled Trials
Overall, there was little difference in the incidence of discontinuation due to adverse events between aripiprazole-treated (7%) and placebo-treated (9%) patients. The types of adverse events that led to discontinuation were similar between the aripiprazole and placebo-treated patients.
Commonly Observed Adverse Events in Short-Term, Placebo-Controlled Trials of Patients with Schizophrenia
The only commonly observed adverse event associated with the use of aripiprazole in patients with schizophrenia (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) was akathisia (placebo 4%; aripiprazole 8%).
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials of Patients with Bipolar Mania
The following findings are based on a pool of 3-week, placebo-controlled, bipolar mania trials in which oral aripiprazole was administered at doses of 15 or 30 mg/day.
Adverse Events Associated with Discontinuation of Treatment in Short-Term, Placebo-Controlled Trials
Overall, in patients with bipolar mania, there was little difference in the incidence of discontinuation due to adverse events between aripiprazole-treated (11%) and placebo-treated (9%) patients. The types of adverse events that led to discontinuation were similar between the aripiprazole and placebo-treated patients.
Commonly Observed Adverse Events in Short-Term, Placebo-Controlled Trials of Patients with Bipolar Mania
Commonly observed adverse events associated with the use of aripiprazole in patients with bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) are shown in Table 1.
| Percentage of Patients Reporting Event | ||
|---|---|---|
Preferred Term |
Aripiprazole (n=597) |
Placebo (n=436) |
| Constipation | 13 | 6 |
| Akathisia | 15 | 3 |
| Sedation | 8 | 3 |
| Tremor | 7 | 3 |
| Restlessness | 6 | 3 |
| Extrapyramidal Disorder | 5 | 2 |
Adverse Events Occurring at an Incidence of 2% or More Among Aripiprazole-Treated Patients and Greater than Placebo in Short-Term, Placebo-Controlled Trials
Table 2 enumerates the pooled incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in bipolar mania), including only those events that occurred in 2% or more of patients treated with aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with aripiprazole was greater than the incidence in patients treated with placebo in the combined dataset.
| Percentage of Patients Reporting Eventa | ||
|---|---|---|
| System Organ Class Preferred Term |
Aripiprazole (n=1523) |
Placebo (n=849) |
| a Events reported by at least 2% of patients treated with oral aripiprazole, except the following events, which had an incidence equal to or less than placebo: diarrhea, toothache, upper abdominal pain, abdominal pain, musculoskeletal stiffness, back pain, myalgia, agitation, psychotic disorder, dysmenorrheaf, rash. | ||
| b Including blood pressure increased. | ||
| f Percentage based on gender total. | ||
| Eye Disorders | ||
| Vision Blurred | 3 | 1 |
| Gastrointestinal Disorders | ||
| Nausea | 16 | 12 |
| Vomiting | 12 | 6 |
| Constipation | 11 | 7 |
| Dyspepsia | 10 | 8 |
| Dry Mouth | 5 | 4 |
| Abdominal Discomfort | 3 | 2 |
| Stomach Discomfort | 3 | 2 |
| Salivary Hypersecretion | 2 | 1 |
| General Disorders and Administration Site Conditions | ||
| Fatigue | 6 | 5 |
| Pain | 3 | 2 |
| Peripheral Edema | 2 | 1 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Arthralgia | 5 | 4 |
| Pain in Extremity | 4 | 2 |
| Nervous System Disorders | ||
| Headache | 30 | 25 |
| Dizziness | 11 | 8 |
| Akathisia | 10 | 4 |
| Sedation | 7 | 4 |
| Extrapyramidal Disorder | 6 | 4 |
| Tremor | 5 | 3 |
| Somnolence | 5 | 4 |
| Psychiatric Disorders | ||
| Anxiety | 20 | 17 |
| Insomnia | 19 | 14 |
| Restlessness | 5 | 3 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||
| Pharyngolaryngeal Pain | 4 | 3 |
| Cough | 3 | 2 |
| Nasal Congestion | 3 | 2 |
| Vascular Disorders | ||
| Hypertensionb | 2 | 1 |
An examination of population subgroups did not reveal any clear evidence of differential adverse event incidence on the basis of age, gender, or race.
INTRAMUSCULAR ADMINISTRATION
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials of Patients with Agitation Associated with Schizophrenia or Bipolar Mania
The following findings are based on a pool of three placebo-controlled trials of patients with agitation associated with schizophrenia or bipolar mania in which aripiprazole injection was administered at doses of 5.25 mg to 15 mg.
Adverse Events Associated with Discontinuation of Treatment in Short-Term, Placebo-Controlled Trials
Overall, in patients with agitation associated with schizophrenia or bipolar mania, there was little difference in the incidence of discontinuation due to adverse events between aripiprazole-treated (0.8%) and placebo-treated (0.5%) patients.
Commonly Observed Adverse Events in Short-Term, Placebo-Controlled Trials of Patients with Agitation Associated with Schizophrenia or Bipolar Mania
There was one commonly observed adverse event (nausea) associated with the use of aripiprazole injection in patients with agitation associated with schizophrenia and bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo).
Adverse Events Occurring at an Incidence of 1% or More Among Aripiprazole-Treated Patients and Greater than Placebo in Short-Term, Placebo-Controlled Trials of Patients with Agitation Associated with Schizophrenia or Bipolar Mania
Table 3 enumerates the pooled incidence, rounded to the nearest percent, of treatment-emergent adverse events that occurred during acute therapy (24 hour), including only those events that occurred in 1% or more of patients treated with aripiprazole injection (doses ≥5.25 mg/day) and for which the incidence in patients treated with aripiprazole injection was greater than the incidence in patients treated with placebo in the combined dataset.
| Percentage of Patients Reporting Eventa | ||
|---|---|---|
| System Organ Class Preferred Term |
Aripiprazole (n=501) |
Placebo (n=220) |
| aEvents reported by at least 1% of patients treated with aripiprazole injection, except the following events, which had an incidence equal to or less than placebo: injection site pain, injection site burning, insomnia, agitation. | ||
| Cardiac Disorders | ||
| Tachycardia | 2 | <1 |
| Gastrointestinal Disorders | ||
| Nausea | 9 | 3 |
| Vomiting | 3 | 1 |
| Dyspepsia | 1 | <1 |
| Dry Mouth | 1 | <1 |
| General Disorders and Administration Site Conditions | ||
| Fatigue | 2 | 1 |
| Investigations | ||
| Blood Pressure Increased | 1 | <1 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal Stiffness | 1 | <1 |
| Nervous System Disorders | ||
| Headache | 12 | 7 |
| Dizziness | 8 | 5 |
| Somnolence | 7 | 4 |
| Sedation | 3 | 2 |
| Akathisia | 2 | 0 |
Dose-Related Adverse Events
Schizophrenia
Dose response relationships for the incidence of treatment-emergent adverse events were evaluated from four trials in patients with schizophrenia comparing various fixed doses (2, 5, 10, 15, 20, and 30 mg/day) of oral aripiprazole to placebo. This analysis, stratified by study, indicated that the only adverse event to have a possible dose response relationship, and then most prominent only with 30 mg, was somnolence ([including sedation] placebo, 7.1%; 10 mg, 8.5%; 15 mg, 8.7%; 20 mg, 7.5%; 30 mg, 12.6%).
Extrapyramidal Symptoms
In the short-term, placebo-controlled trials of schizophrenia, the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 13% vs. 12% for placebo. In the short-term, placebo-controlled trials in schizophrenia, the incidence of akathisia-related events for aripiprazole-treated patients was 8% vs. 4% for placebo. In the short-term, placebo-controlled trials in bipolar mania, the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 15% vs. 8% for placebo. In the short-term, placebo-controlled trials in bipolar mania, the incidence of akathisia-related events for aripiprazole-treated patients was 15% vs. 4% for placebo. Objectively collected data from those trials was collected on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia) and the Assessments of Involuntary Movement Scales (for dyskinesias). In the schizophrenia trials, the objectively collected data did not show a difference between aripiprazole and placebo, with the exception of the Barnes Akathisia Scale (aripiprazole, 0.08; placebo, -0.05). In the bipolar mania trials, the Simpson Angus Rating Scale and the Barnes Akathisia Scale showed a significant difference between aripiprazole and placebo (aripiprazole, 0.61; placebo, 0.03 and aripiprazole, 0.25; placebo, -0.06). Changes in the Assessments of Involuntary Movement Scales were similar for the aripiprazole and placebo groups.
Similarly, in a long-term (26-week), placebo-controlled trial of schizophrenia, objectively collected data on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia), and the Assessments of Involuntary Movement Scales (for dyskinesias) did not show a difference between aripiprazole and placebo.
In the placebo-controlled trials in patients with agitation associated with schizophrenia or bipolar mania, the incidence of reported EPS-related events excluding events related to akathisia for aripiprazole-treated patients was 2% vs. 2% for placebo and the incidence of akathisia-related events for aripiprazole-treated patients was 2% vs. 0% for placebo. Objectively collected data on the Simpson Angus Rating Scale (for EPS) and the Barnes Akathisia Scale (for akathisia) for all treatment groups, did not show a difference between aripiprazole and placebo.
Laboratory Test Abnormalities
A between group comparison for 3- to 6-week, placebo-controlled trials revealed no medically important differences between the aripiprazole and placebo groups in the proportions of patients experiencing potentially clinically significant changes in routine serum chemistry, hematology, or urinalysis parameters. Similarly, there were no aripiprazole/placebo differences in the incidence of discontinuations for changes in serum chemistry, hematology, or urinalysis.
In a long-term (26-week), placebo-controlled trial there were no medically important differences between the aripiprazole and placebo patients in the mean change from baseline in prolactin, fasting glucose, triglyceride, HDL, LDL, and total cholesterol measurements.
Weight Gain
In 4- to 6- week trials in schizophrenia, there was a slight difference in mean weight gain between aripiprazole and placebo patients (+0.7 kg vs. -0.05 kg, respectively), and also a difference in the proportion of patients meeting a weight gain criterion of ≥7% of body weight [aripiprazole (8%) compared to placebo (3%)]. In 3-week trials in mania, the mean weight gain for aripiprazole and placebo patients was 0.0 kg vs. -0.2 kg, respectively. The proportion of patients meeting a weight gain criterion of ≥7% of body weight was aripiprazole (3%) compared to placebo (2%).
Table 4 provides the weight change results from a long-term (26-week), placebo-controlled study of aripiprazole, both mean change from baseline and proportions of patients meeting a weight gain criterion of ≥7% of body weight relative to baseline, categorized by BMI at baseline:
| BMI <23 | BMI 23-27 | BMI >27 | ||||
| Placebo | Aripiprazole | Placebo | Aripiprazole | Placebo | Aripiprazole | |
| Mean change from baseline (kg) |
-0.5 | -0.5 | -0.6 | -1.3 | -1.5 | -2.1 |
| % with ≥7% increase BW | 3.7% | 6.8% | 4.2% | 5.1% | 4.1% | 5.7% |
Table 5 provides the weight change results from a long-term (52-week) study of aripiprazole, both mean change from baseline and proportions of patients meeting a weight gain criterion of ≥7% of body weight relative to baseline, categorized by BMI at baseline:
| BMI <23 | BMI 23-27 | BMI >27 | |
| Mean change from baseline (kg) | 2.6 | 1.4 | -1.2 |
| % with ≥7% increase BW | 30% | 19% | 8% |
ECG Changes
Between group comparisons for a pooled analysis of placebo-controlled trials in patients with schizophrenia or bipolar mania, revealed no significant differences between oral aripiprazole and placebo in the proportion of patients experiencing potentially important changes in ECG parameters. Aripiprazole was associated with a median increase in heart rate of 5 beats per minute compared to a 1 beat per minute increase among placebo patients.
In the pooled, placebo-controlled trials in patients with agitation associated with schizophrenia or bipolar mania, there were no significant differences between aripiprazole injection and placebo in the proportion of patients experiencing potentially important changes in ECG parameters, as measured by standard 12-lead ECGs.
Additional Findings Observed in Clinical Trials
Adverse Events in Long-Term, Double-Blind, Placebo-Controlled Trials
The adverse events reported in a 26-week, double-blind trial comparing oral ABILIFY and placebo in patients with schizophrenia were generally consistent with those reported in the short-term, placebo-controlled trials, except for a higher incidence of tremor [8% (12/153) for ABILIFY vs. 2% (3/153) for placebo]. In this study, the majority of the cases of tremor were of mild intensity (8/12 mild and 4/12 moderate), occurred early in therapy (9/12 ≤49 days), and were of limited duration (7/12 ≤10 days). Tremor infrequently led to discontinuation (<1%) of ABILIFY. In addition, in a long-term (52-week), active-controlled study, the incidence of tremor for ABILIFY was 5% (40/859). A similar adverse event profile was observed in a long-term study in bipolar disorder.
Other Adverse Events Observed During the Premarketing Evaluation of Oral Aripiprazole
Following is a list of MedDRA terms that reflect treatment-emergent adverse events as defined in the introduction to the ADVERSE REACTIONS section reported by patients treated with oral aripiprazole at multiple doses ≥2 mg/day during any phase of a trial within the database of 8456 patients. All reported events are included except those already listed in Table 2, or other parts of the ADVERSE REACTIONS section, those considered in the WARNINGS or PRECAUTIONS, those event terms which were so general as to be uninformative, events reported with an incidence of ≤0.05% and which did not have a substantial probability of being acutely life-threatening, events that are otherwise common as background events, and events considered unlikely to be drug related. It is important to emphasize that, although the events reported occurred during treatment with aripiprazole, they were not necessarily caused by it.
Events are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring in at least 1/100 patients (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing); infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Blood and Lymphatic System Disorders: Infrequent - anaemia, lymphadenopathy, leukopenia (including agranulocytosis, neutropenia); Rare - leukocytosis, thrombocytopenia, idiopathic thrombocytopenic purpura, thrombocythaemia.
Cardiac Disorders: Frequent - tachycardia (including ventricular, supraventricular, sinus); Infrequent -bradycardia, palpitations, cardiac failure (including congestive and acute), myocardial infarction, cardiac arrest, atrial fibrillation, atrioventricular block (including first degree and complete), extrasystoles (including ventricular and supraventricular), angina pectoris, cyanosis, bundle branch block (including left, right), myocardial ischaemia; Rare - atrial flutter, cardiomegaly, cardiomyopathy, cardiopulmonary failure.
Ear and Labyrinth Disorders: Infrequent - ear pain, vertigo, tinnitus; Rare - deafness.
Endocrine Disorders: Infrequent - hypothyroidism; Rare - goitre, hyperparathyroidism, hyperthyroidism.
Eye Disorders: Frequent - conjunctivitis; Infrequent - eye redness, eye irritation, dry eye, blepharospasm, visual disturbance, eye pain, eye discharge, blepharitis, cataract, lacrimation increased; Rare - eyelid function disorder, oculogyration, eyelid oedema, photophobia, diplopia, eyelid ptosis, eye haemorrhage.
Gastrointestinal Disorders: Frequent - loose stools; Infrequent - flatulence, dysphagia, gastroesophageal reflux disease, gastritis, haemorrhoids, abdominal distension, faecal incontinence, haematochezia, gingival pain, rectal haemorrhage, abdominal pain lower, oral pain, retching, faecaloma, gastrointestinal haemorrhage, ulcer (including gastric, duodenal, peptic), tooth fracture, gingivitis, lip dry; Rare - abdominal tenderness, chapped lips, periodontitis, aptyalism, gastrointestinal pain, hypoaesthesia oral, inguinal hernia, swollen tongue, colitis, haematemesis, hyperchlorhydria, irritable bowel syndrome, oesophagitis, faeces hard, gingival bleeding, glossodynia, mouth ulceration, reflux oesophagitis, cheilitis, intestinal obstruction, pancreatitis, eructation, gastric ulcer haemorrhage, melaena, glossitis, stomatitis.
General Disorders and Administration Site Conditions: Frequent - asthenia, pyrexia, chest pain, gait disturbance; Infrequent - malaise, oedema, influenza-like illness, chills, general physical health deterioration, feeling jittery, mobility decreased, thirst, feeling cold, difficulty in walking, facial pain, sluggishness, condition aggravated; Rare - inflammation localized, swelling, energy increased, inflammation, abasia, xerosis, feeling hot, hyperthermia, hypothermia.
Hepatobiliary Disorders: Infrequent - cholecystitis (including acute and chronic); Rare - cholelithiasis, hepatitis.
Immune System Disorders: Infrequent - hypersensitivity.
Infections and Infestations: Frequent - respiratory tract infection (including upper and lower), pneumonia; Infrequent - cellulitis, dental caries, vaginitis, vaginal infection, cystitis, vaginal mycosis, eye infection, gastroenteritis, onychomycosis, vaginal candidiasis, otitis media, folliculitis, candidiasis, otitis externa, pyelonephritis, rash pustular; Rare - appendicitis, septic shock.
Injury, Poisoning, and Procedural Complications: Frequent - fall, skin laceration, contusion, fracture; Infrequent - blister, scratch, joint sprain, burn, muscle strain, periorbital haematoma, arthropod bite/sting, head injury, sunburn; Rare - joint dislocation, alcohol poisoning, road traffic accident, self mutilation, eye penetration, injury asphyxiation, poisoning, heat exhaustion, heat stroke.
Investigations: Frequent - weight decreased, blood creatine phosphokinase increased; Infrequent - blood glucose increased, heart rate increased, body temperature increased, alanine aminotransferase increased, blood cholesterol increased, white blood cell count increased, haemoglobin decreased, aspartame aminotransferase increased, blood urea increased, electrocardiogram ST segment abnormal (including depression, elevation), haematocrit decreased, hepatic enzyme increased, blood bilirubin increased, blood glucose decreased, blood creatinine increased, blood alkaline phosphatase increased, blood pressure decreased, blood potassium decreased, blood urine present, electrocardiogram QT corrected interval prolonged; Rare - transaminases increased, blood triglycerides increased, blood uric acid increased, cardiac murmur, eosinophil count increased, neutrophil count increased, platelet count increased, red blood cell count decreased, white blood cell count decreased, white blood cells urine positive, bacteria urine identified, blood lactate dehydrogenase increased, blood potassium increased, neutrophil count decreased, urine output decreased, blood creatine phosphokinase MB increased, ECG signs of myocardial ischemia, electrocardiogram T-wave inversion, heart rate decreased, tuberculin test positive, glucose urine present, glycosylated haemoglobin increased, glucose tolerance decreased, glycosylated haemoglobin decreased, muscle enzyme increased.
Metabolism and Nutrition Disorders: Frequent - decreased appetite (including diet refusal, markedly reduced dietary intake), dehydration; Infrequent - anorexia, increased appetite, hypercholesterolaemia, hypokalaemia, hyperglycaemia, diabetes mellitus, hypoglycaemia, hyponatremia, diabetes mellitus non-insulin-dependent, hyperlipidaemia, obesity (including overweight), polydipsia; Rare - hypertriglyceridaemia, gout, hypernatraemia, weight fluctuation, diabetes mellitus inadequate control.
Musculoskeletal and Connective Tissue Disorders: Frequent - musculoskeletal pain (including neck, jaw, chest wall, bone, buttock, groin, flank, musculoskeletal chest, pubic, and sacral), muscle rigidity, muscle cramp; Infrequent - muscle twitching, joint swelling, muscle spasms, muscle tightness, arthritis, osteoarthritis, muscular weakness, joint range of motion decreased, sensation of heaviness; Rare - tendonitis, osteoporosis, trismus, arthropathy, bursitis, exostosis, night cramps, coccydynia, joint contracture, localised osteoarthritis, osteopenia, rhabdomyolysis, costochondritis, rheumatoid arthritis, torticollis.
Nervous System Disorders: Frequent - lethargy, dyskinesia; Infrequent - disturbance in attention, parkinsonism, dystonia, drooling, cogwheel rigidity, dysarthria, paraesthesia, hypoaesthesia, loss of consciousness (including depressed level of consciousness), hypersomnia, psychomotor hyperactivity, balance disorder, cerebrovascular accident, hypokinesia, tardive dyskinesia, memory impairment, amnesia, ataxia, dementia, hypotonia, burning sensation, dysgeusia, restless leg syndrome, hypertonia, Parkinson’s disease, akinesia, dysphasia, transient ischaemic attack, facial palsy, hemiparesis, myoclonus, sciatica; Rare - bradykinesia, coordination abnormal, cognitive disorder, syncope vasovagal, carpal tunnel syndrome, hyporeflexia, intention tremor, muscle contractions involuntary, sleep apnea syndrome, dementia Alzheimer’s type, epilepsy, hyperreflexia, mastication disorder, mental impairment, nerve compression, parkinsonian gait, tongue paralysis, aphasia, choreoathetosis, formication, masked facies, neuralgia, paresthesia oral, parkinsonian rest tremor, cerebral haemorrhage, dizziness exertional, hyperaesthesia, haemorrhage intracranial, ischaemic stroke, judgment impaired, subarachnoid haemorrhage.
Psychiatric Disorders: Frequent - schizophrenia (including schizoaffective disorder), depression (including depressive symptom), hallucination (including auditory, visual, tactile, mixed, olfactory, and somatic), mood altered (including depressed, euphoric, elevated, and mood swings), paranoia, irritability, suicidal ideation, confusional state, aggression, mania, delusion (including persecutory, perception, somatic, and grandeur); Infrequent - tension, nervousness, nightmare, excitability, panic attack (including panic disorder, panic disorder with agoraphobia, and panic reaction), abnormal dreams, apathy, libido decreased, hostility, suicide attempt, bipolar disorder (including bipolar I), libido increased, anger, delirium, acute psychosis, disorientation, bruxism, hypomania, obsessive-compulsive disorder (including obsessive thoughts), mental status changes, crying, dysphoria, completed suicide, flat affect, impulsive behaviour; Rare - blunted affect, cognitive deterioration, logorrhea, psychomotor agitation, social avoidant behaviour, psychomotor retardation, suspiciousness, affect lability, anorgasmia, fear, homicidal ideation, tic, premature ejaculation, dysphemia, bradyphrenia, derealisation, depersonalisation.
Renal and Urinary Disorders: Infrequent - pollakiuria, dysuria, haematuria, urinary retention, renal failure (including acute and chronic), urinary hesitation, enuresis, nephrolithiasis, micturition urgency, polyuria; Rare - nocturia, proteinuria, glycosuria, calculus urinary, azotaemia.
Reproductive System and Breast Disorders: Infrequent - erectile dysfunction, vaginal discharge, amenorrhoea, vaginal haemorrhage, menstruation irregular, menorrhagia, premenstrual syndrome, testicular pain, genital pruritus female, ovarian cyst, benign prostatic hyperplasia, prostatitis; Rare - gynaecomastia, priapism (including spontaneous penile erection), breast pain, pelvic pain, epididymitis, galactorrhoea, uterine haemorrhage.
Respiratory, Thoracic, and Mediastinal Disorders: Frequent - dyspnoea (including exertional); Infrequent - sinus congestion, rhinorrhoea, wheezing, epistaxis, asthma, hiccups, productive cough, chronic obstructive airways disease (including exacerbated), rhinitis allergic, pneumonia aspiration, pulmonary congestion, sinus pain, respiratory distress, dry throat, hoarseness; Rare- bronchopneumopathy, haemoptysis, respiratory arrest, sneezing, hypoxia, pulmonary embolism, pulmonary oedema (including acute), respiratory failure, brochospasm, nasal dryness, paranasal sinus hypersecretion, pharyngeal erythema, rhonchi, tonsillar hypertrophy, asphyxia, Mendelson’s syndrome.
Skin and Subcutaneous Tissue Disorders: Infrequent - hyperhydrosis, erythema, pruritis (including generalised), dry skin, decubitus ulcer, dermatitis (including allergic, seborrhoeic, acneiform, exfoliative, bullous, neurodermatitis), ecchymosis, skin ulcer, acne, eczema, hyperkeratosis, swelling face, skin discoloration, photosensitivity reaction, skin irritation, alopecia, rash maculopapular, cold sweat, scab, face oedema, dermal cyst, psoriasis, night sweats, rash erythematous; Rare - rash scaly, urticaria, rosacea, seborrhoea, periorbital oedema, rash vesicular.
Vascular Disorders: Frequent - hypotension; Infrequent- hot flush (including flushing), haematoma, deep vein thrombosis, phlebitis; Rare - pallor, petechiae, varicose vein, circulatory collapse, haemorrhage, thrombophlebitis, shock.
Other Adverse Events Observed During the Premarketing Evaluation of Aripiprazole Injection
Following is a list of MedDRA terms that reflect treatment-emergent adverse events as defined in the introduction to the ADVERSE REACTIONS section reported by patients treated with aripiprazole injection at doses ≥1 mg/day during any phase of a trial within the database of 749 patients. All reported events are included except those already listed in Table 2 or 3, or other parts of the ADVERSE REACTIONS section, those considered in the WARNINGS or PRECAUTIONS, those event terms which were so general as to be uninformative, events reported with an incidence of ≤0.05% and which did not have a substantial probability of being acutely life-threatening, events that are otherwise common as background events, and events considered unlikely to be drug related. It is important to emphasize that, although the events reported occurred during treatment with aripiprazole injection, they were not necessarily caused by it.
Events are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring in at least 1/100 patients (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing); infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Ear and Labyrinth Disorders: Infrequent- hyperacusis.
General Disorders and Administration Site Conditions: Infrequent - injection site stinging, abnormal feeling, injection site pruritus, injection site swelling, venipuncture site bruise.
Infections and Infestations: Infrequent - bacteruria, urinary tract infection, urosepsis.
Investigations: Infrequent - blood pressure abnormal, heart rate irregular, electrocardiogram T-wave abnormal.
Psychiatric Disorders: Infrequent - intentional self-injury.
Respiratory, Thoracic, and Mediastinal Disorders: Infrequent - pharyngolaryngeal pain, nasal congestion.
Vascular Disorders: Infrequent - blood pressure fluctuation.
Other Events Observed During the Postmarketing Evaluation of Aripiprazole
Voluntary reports of adverse events in patients taking aripiprazole that have been received since market introduction and not listed above that may have no causal relationship with the drug include rare occurrences of allergic reaction (eg, anaphylactic reaction, angioedema, laryngospasm, oropharyngeal spasm, pruritis, or urticaria), grand mal seizure, and jaundice.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
ABILIFY (aripiprazole) is not a controlled substance.
Abuse and Dependence
Aripiprazole has not been systematically studied in humans for its potential for abuse, tolerance, or physical dependence. In physical dependence studies in monkeys, withdrawal symptoms were observed upon abrupt cessation of dosing. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of ABILIFY misuse or abuse (eg, development of tolerance, increases in dose, drug-seeking behavior).
OVERDOSAGE
MedDRA terminology has been used to classify the adverse events.
Human Experience
A total of 76 cases of deliberate or accidental overdosage with oral aripiprazole have been reported worldwide. These include overdoses with oral aripiprazole alone and in combination with other substances. No fatality was reported from these cases. Of the 44 cases with known outcome, 33 recovered without sequelae and one recovered with sequelae (mydriasis and feeling abnormal). The largest known acute ingestion with a known outcome involved 1080 mg of oral aripiprazole (36 times the maximum recommended daily dose) in a patient who fully recovered. Included in the 76 cases are 10 cases of deliberate or accidental overdosage in children (age 12 and younger) involving oral aripiprazole ingestions up to 195 mg with no fatalities.
Common adverse events (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
Management of Overdosage
No specific information is available on the treatment of overdose with aripiprazole. An electrocardiogram should be obtained in case of overdosage and, if QTc interval prolongation is present, cardiac monitoring should be instituted. Otherwise, management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers.
Charcoal: In the event of an overdose of ABILIFY, an early charcoal administration may be useful in partially preventing the absorption of aripiprazole. Administration of 50 g of activated charcoal, one hour after a single 15-mg oral dose of aripiprazole, decreased the mean AUC and Cmax of aripiprazole by 50%.
Hemodialysis: Although there is no information on the effect of hemodialysis in treating an overdose with aripiprazole, hemodialysis is unlikely to be useful in overdose management since aripiprazole is highly bound to plasma proteins.
DOSAGE AND ADMINISTRATION
ORAL
Schizophrenia
Usual Dose
The recommended starting and target dose for ABILIFY is 10 or 15 mg/day administered on a once-a-day schedule without regard to meals. ABILIFY has been systematically evaluated and shown to be effective in a dose range of 10 to 30 mg/day, when administered as the tablet formulation; however, doses higher than 10 or 15 mg/day were not more effective than 10 or 15 mg/day. Dosage increases should not be made before 2 weeks, the time needed to achieve steady state.
Dosage in Special Populations
Dosage adjustments are not routinely indicated on the basis of age, gender, race, or renal or hepatic impairment status (see CLINICAL PHARMACOLOGY: Special Populations).
Dosage adjustment for patients taking aripiprazole concomitantly with potential CYP3A4 inhibitors: When concomitant administration of ketoconazole with aripiprazole occurs, aripiprazole dose should be reduced to one-half of the usual dose. When the CYP3A4 inhibitor is withdrawn from the combination therapy, aripiprazole dose should then be increased.
Dosage adjustment for patients taking aripiprazole concomitantly with potential CYP2D6 inhibitors: When concomitant administration of potential CYP2D6 inhibitors such as quinidine, fluoxetine, or paroxetine with aripiprazole occurs, aripiprazole dose should be reduced at least to one-half of its normal dose. When the CYP2D6 inhibitor is withdrawn from the combination therapy, aripiprazole dose should then be increased.
Dosage adjustment for patients taking potential CYP3A4 inducers: When a potential CYP3A4 inducer such as carbamazepine is added to aripiprazole therapy, the aripiprazole dose should be doubled (to 20 or 30 mg). Additional dose increases should be based on clinical evaluation. When carbamazepine is withdrawn from the combination therapy, the aripiprazole dose should be reduced to 10 to 15 mg.
Maintenance Therapy
While there is no body of evidence available to answer the question of how long a patient treated with aripiprazole should remain on it, systematic evaluation of patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer, were discontinued from those medications, and were then administered ABILIFY 15 mg/day and observed for relapse during a period of up to 26 weeks, demonstrated a benefit of such maintenance treatment (see CLINICAL PHARMACOLOGY: Clinical Studies). Patients should be periodically reassessed to determine the need for maintenance treatment.
Switching from Other Antipsychotics
There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to ABILIFY or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized.
Bipolar Disorder
Usual Dose
In clinical trials, the starting dose was 30 mg given once a day. A dose of 30 mg/day was found to be effective when administered as the tablet formulation. Approximately 15% of patients had their dose decreased to 15 mg based on assessment of tolerability. The safety of doses above 30 mg/day has not been evaluated in clinical trials.
Dosage in Special Populations
See Dosage in Special Populations under DOSAGE AND ADMINISTRATION: Schizophrenia.
Maintenance Therapy
While there is no body of evidence available to answer the question of how long a patient treated with aripiprazole should remain on it, patients with Bipolar I Disorder who had been symptomatically stable on ABILIFY Tablets (15 mg/day or 30 mg/day with a starting dose of 30 mg/day) for at least 6 consecutive weeks and then randomized to ABILIFY Tablets (15 mg/day or 30 mg/day) or placebo and monitored for relapse, demonstrated a benefit of such maintenance treatment (see CLINICAL PHARMACOLOGY: Clinical Studies). While it is generally agreed that pharmacological treatment beyond an acute response in mania is desirable, both for maintenance of the initial response and for prevention of new manic episodes, there are no systematically obtained data to support the use of aripiprazole in such longer-term treatment (ie, beyond 6 weeks).
Oral Solution
The oral solution can be given on a mg-per-mg basis in place of the 5-, 10-, 15-, or 20-mg tablet strengths. Solution doses can be substituted for the tablet doses on a mg-per-mg basis up to 25 mg of the tablet. Patients receiving 30-mg tablets should receive 25 mg of the solution (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Directions for Use of ABILIFY DISCMELT Orally Disintegrating Tablets
Patients should be told the following:
Do not open the blister until ready to administer. For single tablet removal, open the package and peel back the foil on the blister to expose the tablet. Do not push the tablet through the foil because this could damage the tablet. Immediately upon opening the blister, using dry hands, remove the tablet and place the entire ABILIFY DISCMELT orally disintegrating tablet on the tongue. Tablet disintegration occurs rapidly in saliva. It is recommended that ABILIFY DISCMELT be taken without liquid. However, if needed, it can be taken with liquid. Do not attempt to split the tablet.
INTRAMUSCULAR INJECTION
Agitation Associated with Schizophrenia or Bipolar Mania
Usual Dose
The efficacy of aripiprazole injection in controlling agitation in these disorders was demonstrated in a dose range of 5.25 mg to 15 mg. The recommended dose in these patients is 9.75 mg. No additional benefit was demonstrated for 15 mg compared to 9.75 mg. A lower dose of 5.25 mg may be considered when clinical factors warrant. If agitation warranting a second dose persists following the initial dose, cumulative doses up to a total of 30 mg/day may be given. However, the efficacy of repeated doses of aripiprazole injection in agitated patients has not been systematically evaluated in controlled clinical trials. Also, the safety of total daily doses greater than 30 mg or injections given more frequently than every 2 hours have not been adequately evaluated in clinical trials.
If ongoing aripiprazole therapy is clinically indicated, oral aripiprazole in a range of 10 mg to 30 mg/day should replace aripiprazole injection as soon as possible (see PHARMACOLOGY and DOSAGE AND ADMINISTRATION: Schizophrenia or Bipolar Disorder).
Administration of ABILIFY Injection
To administer ABILIFY Injection, draw up the required volume of solution into the syringe as shown in Table 6. Discard any unused portion.
| Single-Dose | Required Volume of Solution |
|---|---|
| 5.25 mg | 0.7 mL |
| 9.75 mg | 1.3 mL |
| 15 mg | 2 mL |
ABILIFY Injection is intended for intramuscular use only. Do not administer intravenously or subcutaneously. Inject slowly, deep into the muscle mass.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Dosage in Special Populations
See Dosage in Special Populations under DOSAGE AND ADMINISTRATION: Schizophrenia.
ANIMAL TOXICOLOGY
Aripiprazole produced retinal degeneration in albino rats in a 26-week chronic toxicity study at a dose of 60 mg/kg and in a 2-year carcinogenicity study at doses of 40 and 60 mg/kg. The 40- and 60-mg/kg doses are 13 and 19 times the maximum recommended human dose (MRHD) based on mg/m2 and 7 to 14 times human exposure at MRHD based on AUC. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of retinal degeneration. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown.
HOW SUPPLIED
ABILIFY® (aripiprazole) Tablets have markings on one side and are available in the strengths and packages listed in Table 7.
| Tablet Strength |
Tablet Color/Shape |
Tablet Markings |
Pack Size |
NDC Code |
|---|---|---|---|---|
| 2 mg | green modified rectangle |
"A-006" and "2" |
Bottle of 30 Blister of 100 |
59148-006-13 59148-006-35 |
| 5 mg | blue modified rectangle |
"A-007" and "5" |
Bottle of 30 Blister of 100 |
59148-007-13 59148-007-35 |
| 10 mg | pink modified rectangle |
"A-008" and "10" |
Bottle of 30 Blister of 100 |
59148-008-13 59148-008-35 |
| 15 mg | yellow round |
"A-009" and "15" |
Bottle of 30 Blister of 100 |
59148-009-13 59148-009-35 |
| 20 mg | white round |
"A-010" and "20" |
Bottle of 30 Blister of 100 |
59148-010-13 59148-010-35 |
| 30 mg | pink round |
"A-011" and "30" |
Bottle of 30 Blister of 100 |
59148-011-13 59148-011-35 |
ABILIFY® DISCMELT™ (aripiprazole) Orally Disintegrating Tablets are round tablets with markings on either side. ABILIFY DISCMELT is available in the strengths and packages listed in Table 8.
| Tablet Strength |
Tablet Color |
Tablet Markings |
Pack Size |
NDC Code |
|---|---|---|---|---|
| 10 mg | pink (with scattered specks) |
"A" and "640" "10" |
Blister of 30 | 59148-640-23 |
| 15 mg | yellow (with scattered specks) |
"A" and "641" "15" |
Blister of 30 | 59148-641-23 |
ABILIFY® (aripiprazole) Oral Solution (1 mg/mL) is supplied in child-resistant bottles along with a calibrated oral dosing cup. ABILIFY oral solution is available as follows:
150-mL bottle NDC 59148-013-15
ABILIFY® (aripiprazole) Injection for intramuscular use is available as a ready-to-use, 9.75 mg/1.3 mL (7.5 mg/mL) solution in clear, Type 1 glass vials as follows:
9.75 mg/1.3 mL single-dose vial NDC 59148-016-65
Storage
Tablets
Store at 25° C (77° F); excursions permitted between 15° C to 30° C (59° F to 86° F) [see USP Controlled Room Temperature].
Oral Solution
Store at 25° C (77° F); excursions permitted between 15° C to 30° C (59° F to 86° F) [see USP Controlled Room Temperature]. Opened bottles of ABILIFY oral solution can be used for up to 6 months after opening, but not beyond the expiration date on the bottle. The bottle and its contents should be discarded after the expiration date.
Injection
Store at 25° C (77° F); excursions permitted between 15° C to 30° C (59° F to 86° F) [see USP Controlled Room Temperature]. Protect from light by storing in the original container. Retain in carton until time of use.
Tablets manufactured by Otsuka Pharmaceutical Co, Ltd, Tokyo, 101-8535 Japan or Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
Orally disintegrating tablets, Oral solution and Injection manufactured by Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
Distributed and marketed by Otsuka America Pharmaceutical, Inc, Rockville, MD 20850 USA
Marketed by Bristol-Myers Squibb Company, Princeton, NJ 08543 USA
US Patent Nos: 5,006,528; 6,977,257; and 7,115,587
© 2006, Otsuka Pharmaceutical Co, Ltd, Tokyo, 101-8535 Japan
1216978A2
191707A8
1174/11-06
Rev November 2006
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| ABILIFY DISCMELT (aripiprazole) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABILIFY DISCMELT (aripiprazole) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| ABILIFY (aripiprazole) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 02/2007Otsuka Pharmaceutical Co, Ltd