sterile water (Water) irrigant
[B. Braun Medical Inc.]
DESCRIPTION
Sterile Water for Irrigation USP is a sterile, hypotonic, nonpyrogenic irrigating fluid or pharmaceutic aid (solvent) entirely composed of Sterile Water for Injection USP. It is prepared by distillation and contains no antimicrobial or bacteriostatic agents or added buffers. The pH is 5.7 (5.0–7.0)
The plastic container is a copolymer of ethylene and propylene formulated and developed for parenteral drugs. The copolymer contains no plasticizers and exhibits virtually no leachability. The plastic container is also virtually impermeable to vapor transmission and therefore, requires no overwrap to maintain the proper drug concentration. The safety of the plastic container has been confirmed by biological evaluation procedures. The material passes Class Vl testing as specified in the U.S. Pharmacopeia for Biological Tests—Plastic Containers. These tests have shown that the container is nontoxic and biologically inert.
CLINICAL PHARMACOLOGY
Sterile Water for Irrigation USP is utilized for a variety of clinical indications. Because of its low refractive index (1.3325), water provides excellent visibility during endoscopic urological procedures. It is also utilized as a pharmaceutic aid, as well as in the preparation of enteral nutrient products.
Water is hypotonic and will cause hemolysis and will be readily absorbed by the tissues during surgical procedures; therefore, its use under such conditions is not recommended.
INDICATIONS AND USAGE
Sterile Water for Irrigation USP is indicated for use as an irrigating fluid or pharmaceutic aid. Sterile Water may also be used as an adjunct in the preparation of non-intravenously administered nutrient mixtures (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
Not for injection.
WARNINGS
Sterile Water for Irrigation USP is hypotonic and will cause hemolysis, and is not recommended for use during surgical procedures.
After opening container, its contents should be used promptly to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservative.
PRECAUTIONS
Use only if solution is clear and container and seal are intact.
ADVERSE REACTIONS
None known.
OVERDOSAGE
None known.
DOSAGE AND ADMINISTRATION
Irrigation
Use as directed by physician.
This drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Nutrient Mixtures
Sterile Water for Irrigation USP may be used to prepare non-intravenously administered nutrient mixtures. It contains no electrolytes or other added substances. Refer to preparation instructions of particular mixture to be used. The plastic container may be used for administration of non-intravenous nutrient mixture to the patient as appropriate.
HOW SUPPLIED
Sterile Water for Irrigation USP is supplied sterile and nonpyrogenic in plastic irrigating containers. The 1000 mL and 500 mL containers are packaged 16 per case; the 2000 mL are packaged 8 per case and the 4000 mL are packaged 4 per case.
| NDC | Cat. No. | Size |
|---|---|---|
| Sterile Water for Irrigation USP (Canada DIN 01963961) | ||
| 0264-2101-00 | R5000-01 | 1000 mL |
| 0264-2101-10 | R5001-01 | 500 mL |
| 0264-2101-50 | R5005-01 | 2000 mL |
| 0264-2101-70 | R5007 | 4000 mL |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Do not warm above 150°F (66°C).
Rx only
Rev: January 2003
Directions For Use
Aseptic technique is required.
- Caution – Before use, perform the following checks:
- (a)
- Read the label. Ensure solution is the one ordered and is within the expiration date.
- (b)
- Invert container and inspect the solution in good light for cloudiness, haze or particulate matter; check the container for leakage or damage. Any container which is suspect should not be used.
Use only if solution is clear and container and seal are intact.
Single unit container.
Discard unused portion.
Not for injection.
- 2.
- Outer Closure Removal – Grasp the container with one hand and turn the breakaway ring counterclockwise with the other hand until slight resistance is felt. Then, twisting the container in the opposite direction, turn the breakaway ring sharply until the entire outer cap is loose and can be lifted off.
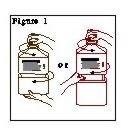
- 3.
- Connect the administration set through the sterile set port according to set instructions or remove screw cap and pour.
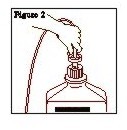
- 4.
- Do not warm above 150°F to assure minimal bottle distortion. Keep bottles upright.
Notice: An additional suspension device has been added to the container hanger tab on the 4000 mL container. Please use this device to suspend the container to permit twisting without the potential for breakage of the integral hanger.
B. Braun Medical Inc.
Irvine, CA USA 92614-5895
In Canada, distributed by:
B. Braun Medical Inc.
Scarborough, Ontario M1H 2W4
©2003 B. Braun Medical Inc.
Y36-002-474
| Sterile Water (Water) | |||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Revised: 12/2006