clonidine hydrochloride (Clonidine Hydrochloride) tablet
[Mylan Pharmaceuticals Inc.]
0.1 mg, 0.2 mg and 0.3 mg
Rx only
DESCRIPTION
Clonidine hydrochloride is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base.
Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-(2,6-dichlorophenylamino)-2- imidazoline hydrochloride. The following is the structural formula:
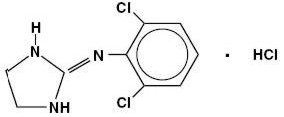
C9H9Cl2N3• HCl
M.W. 266.56
Clonidine hydrochloride is an odorless, bitter, white, crystalline substance soluble in water and alcohol.
Each tablet for oral administration contains ammonium chloride, colloidal silicon dioxide, croscarmellose sodium (Type A), magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Clonidine stimulates alpha-adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal vascular resistance, heart rate, and blood pressure. Clonidine hydrochloride acts relatively rapidly. The patient's blood pressure declines within 30 to 60 minutes after an oral dose, the maximum decrease occurring within 2 to 4 hours. Renal blood flow and glomerular filtration rate remain essentially unchanged. Normal postural reflexes are intact; therefore, orthostatic symptoms are mild and infrequent.
Acute studies with clonidine hydrochloride in humans have demonstrated a moderate reduction (15% to 20%) of cardiac output in the supine position with no change in the peripheral resistance: at a 45° tilt there is a smaller reduction in cardiac output and a decrease of peripheral resistance. During long term therapy, cardiac output tends to return to control values, while peripheral resistance remains decreased. Slowing of the pulse rate has been observed in most patients given clonidine, but the drug does not alter normal hemodynamic response to exercise.
Tolerance to the antihypertensive effect may develop in some patients, necessitating a reevaluation of therapy.
Other studies in patients have provided evidence of a reduction in plasma renin activity and in the excretion of aldosterone and catecholamines. The exact relationship of these pharmacologic actions to the antihypertensive effect of clonidine has not been fully elucidated.
Clonidine acutely stimulates growth hormone release in both children and adults, but does not produce a chronic elevation of growth hormone with long-term use.
Pharmacokinetics
The plasma level of clonidine peaks in approximately 3 to 5 hours and the plasma half-life ranges from 12 to 16 hours. The half-life increases up to 41 hours in patients with severe impairment of renal function. Following oral administration about 40–60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. About 50% of the absorbed dose is metabolized in the liver.
INDICATIONS AND USAGE
Clonidine hydrochloride is indicated in the treatment of hypertension. Clonidine hydrochloride may be employed alone or concomitantly with other antihypertensive agents.
CONTRAINDICATIONS
Clonidine hydrochloride tablets should not be used in patients with known hypersensitivity to clonidine (see PRECAUTIONS).
WARNINGS
Withdrawal
Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor accompanied or followed by a rapid rise in blood pressure and elevated catecholamine concentrations in the plasma. The likelihood of such reactions to discontinuation of clonidine therapy appears to be greater after administration of higher doses or continuation of concomitant beta-blocker treatment and special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after clonidine withdrawal. When discontinuing therapy with clonidine hydrochloride, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptomatology.
An excessive rise in blood pressure following discontinuation of clonidine hydrochloride therapy can be reversed by administration of oral clonidine hydrochloride or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of clonidine hydrochloride.
Because children commonly have gastrointestinal illnesses that lead to vomiting, they may be particularly susceptible to hypertensive episodes resulting from abrupt inability to take medication.
PRECAUTIONS
General
In patients who have developed localized contact sensitization to transdermal clonidine, substitution of oral clonidine hydrochloride therapy may be associated with the development of a generalized skin rash.
In patients who develop an allergic reaction to transdermal clonidine, substitution of oral clonidine hydrochloride may also elicit an allergic reaction (including generalized rash, urticaria, or angioedema).
Clonidine hydrochloride should be used with caution in patients with severe coronary insufficiency, conduction disturbances, recent myocardial infarction, cerebrovascular disease or chronic renal failure.
Perioperative Use
Administration of clonidine hydrochloride should be continued to within four hours of surgery and resumed as soon as possible thereafter. Blood pressure should be carefully monitored during surgery and additional measures to control blood pressure should be available if required.
Information for Patients
Patients should be cautioned against interruption of clonidine therapy without their physician's advice.
Patients who engage in potentially hazardous activities, such as operating machinery or driving, should be advised of a possible sedative effect of clonidine. They should also be informed that this sedative effect may be increased by concomitant use of alcohol, barbiturates, or other sedating drugs.
Drug Interactions
Clonidine may potentiate the CNS-depressive effects of alcohol, barbiturates or other sedatives. If a patient receiving clonidine hydrochloride is also taking tricyclic antidepressants, the hypotensive effect of clonidine may be reduced, necessitating an increase in the clonidine dosage.
Due to a potential for additive effects such as bradycardia and AV block, caution is warranted in patients receiving clonidine concomitantly with agents known to affect sinus node function or AV nodal conduction, e.g. digitalis, calcium channel blockers and beta-blockers.
Amitriptyline in combination with clonidine enhances the manifestation of corneal lesions in rats (see TOXICOLOGY).
Toxicology
In several studies with oral clonidine hydrochloride, a dose-dependent increase in the incidence and severity of spontaneous retinal degeneration was seen in albino rats treated for six months or longer. Tissue distribution studies in dogs and monkeys showed a concentration of clonidine in the choroid.
In view of the retinal degeneration seen in rats, eye examinations were performed during clinical trials in 908 patients before, and periodically after, the start of clonidine therapy. In 353 of these 908 patients, the eye examinations were carried out over periods of 24 months or longer. Except for some dryness of the eyes, no drug-related abnormal ophthalmological findings were recorded and, according to specialized tests such as electroretinography and macular dazzle, retinal function was unchanged.
In combination with amitriptyline, clonidine hydrochloride administration led to the development of corneal lesions in rats within 5 days.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Chronic dietary administration of clonidine was not carcinogenic to rats (132 weeks) or mice (78 weeks) dosed, respectively, at up to 46 or 70 times the maximum recommended daily human dose as mg/kg (9 or 6 times the MRDHD on a mg/m2 basis). There was no evidence of genotoxicity in the Ames test for mutagenicity or mouse micronucleus test for clastogenicity.
Fertility of male or female rats was unaffected by clonidine doses as high as 150 mcg/kg (about 3 times the MRDHD). In a separate experiment, fertility of female rats appeared to be affected at dose levels of 500 to 2000 mcg/kg (10 to 40 times the oral MRDHD on a mg/kg basis; 2 to 8 times the MRDHD on a mg/m2 basis).
Usage in Pregnancy
Teratogenic Effects
Pregnancy Category C
Reproduction studies performed in rabbits at doses up to approximately 3 times the oral maximum recommended daily human dose (MRDHD) of clonidine hydrochloride produced no evidence of teratogenic or embryotoxic potential in rabbits. In rats, however, doses as low as 1/3 the oral MRDHD (1/15 the MRDHD on a mg/m2 basis) of clonidine were associated with increased resorptions in a study in which dams were treated continuously from 2 months prior to mating. Increased resorptions were not associated with treatment at the same or at higher dose levels (up to 3 times the oral MRDHD) when dams were treated on gestation days 6–15. Increases in resorptions were observed at much higher dose levels (40 times the oral MRDHD on a mg/kg basis; 4 to 8 times the MRDHD on a mg/m2 basis) in mice and rats treated on gestation days 1–14 (lowest dose employed in that study was 500 mcg/kg).
No adequate, well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
As clonidine hydrochloride is excreted in human milk, caution should be exercised when clonidine hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of twelve have not been established (see Warnings on Withdrawal).
ADVERSE REACTIONS
Most adverse effects are mild and tend to diminish with continued therapy. The most frequent (which appear to be dose-related) are dry mouth, occurring in about 40 of 100 patients; drowsiness, about 33 in 100; dizziness, about 16 in 100; constipation and sedation, each about 10 in 100.
The following less frequent adverse experiences have also been reported in patients receiving clonidine hydrochloride, but in many cases patients were receiving concomitant medication and causal relationship has not been established.
Body as a Whole: Weakness, about 10 in 100 patients; fatigue, about 4 in 100; headache and withdrawal syndrome each about 1 in 100. Also reported were pallor; a weakly positive Coombs' test; increased sensitivity to alcohol; and fever.
Cardiovascular: Orthostatic symptoms, about 3 in 100 patients; palpitations and tachycardia, and bradycardia, each about 5 in 1000. Syncope, Raynaud's phenomenon, congestive heart failure, and electrocardiographic abnormalities (i.e. sinus node arrest, functional bradycardia, high degree AV block and arrhythmias) have been reported rarely. Rare cases of sinus bradycardia and atrioventricular block have been reported, both with and without the use of concomitant digitalis.
Central Nervous System: Nervousness and agitation, about 3 in 100 patients; mental depression, about 1 in 100 and insomnia, about 5 in 1000. Other behavioral changes, vivid dreams or nightmares, restlessness, anxiety, visual and auditory hallucinations and delirium have rarely been reported.
Dermatological: Rash, about 1 in 100 patients; pruritus, about 7 in 1000; hives, angioneurotic edema and urticaria, about 5 in 1000; alopecia, about 2 in 1000.
Gastrointestinal: Nausea and vomiting, about 5 in 100 patients; anorexia and malaise, each about 1 in 100; mild transient abnormalities in liver function tests, about 1 in 100; hepatitis, parotitis, constipation, pseudo-obstruction, and abdominal pain, rarely.
Genitourinary: Decreased sexual activity, impotence and loss of libido, about 3 in 100 patients; nocturia, about 1 in 100; difficulty in micturition, about 2 in 1000; urinary retention, about 1 in 1000.
Hematologic: Thrombocytopenia, rarely.
Metabolic: Weight gain, about 1 in 100 patients; gynecomastia, about 1 in 1000; transient elevation of blood glucose or serum creatine phosphokinase, rarely.
Musculoskeletal: Muscle or joint pain, about 6 in 1000 and leg cramps, about 3 in 1000.
Oro-otolaryngeal: Dryness of the nasal mucosa was rarely reported.
Ophthalmological: Dryness of eyes, burning of the eyes, and blurred vision were reported.
OVERDOSAGE
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis. The frequency of CNS depression may be higher in children than adults. Large overdoses may result in reversible cardiac conduction defects or dysrhythmias, apnea, coma and seizures. Signs and symptoms of overdose generally occur within 30 minutes to two hours after exposure. As little as 0.1 mg of clonidine has produced signs of toxicity in children.
There is no specific antidote for clonidine overdosage. Clonidine overdosage may result in the rapid development of CNS depression; therefore, induction of vomiting with ipecac syrup is not recommended. Gastric lavage may be indicated following recent and/or large ingestions. Administration of activated charcoal and/or a cathartic may be beneficial. Supportive care may include atropine sulfate for bradycardia, intravenous fluids and/or vasopressor agents for hypotension and vasodilators for hypertension. Naloxone may be a useful adjunct for the management of clonidine-induced respiratory depression, hypotension and/or coma; blood pressure should be monitored since the administration of naloxone has occasionally resulted in paradoxical hypertension. Tolazoline administration has yielded inconsistent results and is not recommended as first-line therapy. Dialysis is not likely to significantly enhance the elimination of clonidine.
The largest overdose reported to date involved a 28-year old male who ingested 100 mg of clonidine hydrochloride powder. This patient developed hypertension followed by hypotension, bradycardia, apnea, hallucinations, semicoma, and premature ventricular contractions. The patient fully recovered after intensive treatment. Plasma clonidine levels were 60 ng/mL after 1 hour, 190 ng/mL after 1.5 hours, 370 ng/mL after 2 hours, and 120 ng/mL after 5.5 and 6.5 hours. In mice and rats, the oral LD50 of clonidine is 206 and 465 mg/kg, respectively.
DOSAGE AND ADMINISTRATION
Adults
The dose of clonidine hydrochloride must be adjusted according to the patient's individual blood pressure response. The following is a general guide to its administration.
Initial Dose
0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose.
Maintenance Dose
Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses. Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed.
Renal Impairment
Dosage must be adjusted according to the degree of impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
HOW SUPPLIED
Clonidine hydrochloride tablets, USP are available containing 0.1 mg, 0.2 mg or 0.3 mg of clonidine hydrochloride, USP.
The 0.1 mg tablets are white, round scored tablets debossed with MYLAN 152. They are available as follows:
NDC 0378-0152-01
bottles of 100 tablets
NDC 0378-0152-10
bottles of 1000 tablets
The 0.2 mg tablets are white, round scored tablets debossed with MYLAN 186. They are available as follows:
NDC 0378-0186-01
bottles of 100 tablets
NDC 0378-0186-10
bottles of 1000 tablets
The 0.3 mg tablets are white, round scored tablets debossed with MYLAN 199. They are available as follows:
NDC 0378-0199-01
bottles of 100 tablets
STORE AT CONTROLLED ROOM TEMPERATURE 15°–30°C (59°–86°F).
PROTECT FROM LIGHT.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REV OCTOBER 2002
CLON:R13
| Clonidine Hydrochloride (Clonidine Hydrochloride) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Clonidine Hydrochloride (Clonidine Hydrochloride) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Clonidine Hydrochloride (Clonidine Hydrochloride) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 12/2006