PARAPLATIN
-
carboplatin injection, powder, lyophilized, for solution
E.R. Squibb & Sons, L.L.C.
----------
PARAPLATIN®(carboplatin) for Injection, USP
WARNING
PARAPLATIN (carboplatin) for Injection, USP should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available.
Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug-related side effect.
Anaphylactic-like reactions to PARAPLATIN have been reported and may occur within minutes of PARAPLATIN administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms.
DESCRIPTION
PARAPLATIN® (carboplatin) for Injection, USP is supplied as a sterile, lyophilized white powder available in single-dose vials containing 50 mg, 150 mg, and 450 mg of carboplatin for administration by intravenous infusion. Each vial contains equal parts by weight of carboplatin and mannitol.
Carboplatin is a platinum coordination compound that is used as a cancer chemotherapeutic agent. The chemical name for carboplatin is platinum, diammine[1,1-cyclobutanedicarboxylato(2-)-O,O′]-, (SP-4-2), and has the following structural formula:
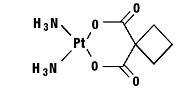
Carboplatin is a crystalline powder with the molecular formula of C6H12N2O4Pt and a molecular weight of 371.25. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5 to 7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide.
CLINICAL PHARMACOLOGY
Carboplatin, like cisplatin, produces predominantly interstrand DNA cross-links rather than DNA-protein cross-links. This effect is apparently cell-cycle nonspecific. The aquation of carboplatin, which is thought to produce the active species, occurs at a slower rate than in the case of cisplatin. Despite this difference, it appears that both carboplatin and cisplatin induce equal numbers of drug-DNA cross-links, causing equivalent lesions and biological effects. The differences in potencies for carboplatin and cisplatin appear to be directly related to the difference in aquation rates.
In patients with creatinine clearances of about 60 mL/min or greater, plasma levels of intact carboplatin decay in a biphasic manner after a 30-minute intravenous infusion of 300 mg/m2 to 500 mg/m2 of PARAPLATIN. The initial plasma half-life (alpha) was found to be 1.1 to 2 hours (n=6), and the postdistribution plasma half-life (beta) was found to be 2.6 to 5.9 hours (n=6). The total body clearance, apparent volume of distribution and mean residence time for carboplatin are 4.4 L/hour, 16 L and 3.5 hours, respectively. The Cmax values and areas under the plasma concentration versus time curves from 0 to infinity (AUC inf) increase linearly with dose, although the increase was slightly more than dose proportional. Carboplatin, therefore, exhibits linear pharmacokinetics over the dosing range studied (300 mg/m2 - 500 mg/m2).
Carboplatin is not bound to plasma proteins. No significant quantities of protein-free, ultrafilterable platinum-containing species other than carboplatin are present in plasma. However, platinum from carboplatin becomes irreversibly bound to plasma proteins and is slowly eliminated with a minimum half-life of 5 days.
The major route of elimination of carboplatin is renal excretion. Patients with creatinine clearances of approximately 60 mL/min or greater excrete 65% of the dose in the urine within 12 hours and 71% of the dose within 24 hours. All of the platinum in the 24-hour urine is present as carboplatin. Only 3% to 5% of the administered platinum is excreted in the urine between 24 and 96 hours. There are insufficient data to determine whether biliary excretion occurs.
In patients with creatinine clearances below 60 mL/min the total body and renal clearances of carboplatin decrease as the creatinine clearance decreases. PARAPLATIN dosages should, therefore, be reduced in these patients (see DOSAGE AND ADMINISTRATION).
The primary determinant of PARAPLATIN clearance is glomerular filtration rate (GFR) and this parameter of renal function is often decreased in elderly patients. Dosing formulas incorporating estimates of GFR (see DOSAGE AND ADMINISTRATION) to provide predictable PARAPLATIN plasma AUCs should be used in elderly patients to minimize the risk of toxicity.
CLINICAL STUDIES
Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer
In two prospectively randomized, controlled studies conducted by the National Cancer Institute of Canada, Clinical Trials Group (NCIC), and the Southwest Oncology Group (SWOG), 789 chemotherapy naive patients with advanced ovarian cancer were treated with PARAPLATIN or cisplatin, both in combination with cyclophosphamide every 28 days for six courses before surgical reevaluation. The following results were obtained from both studies:
Comparative Efficacy
| NCIC | SWOG | |
|---|---|---|
| Number of patients randomized | 447 | 342 |
| Median age (years) | 60 | 62 |
| Dose of cisplatin | 75 mg/m2 | 100 mg/m2 |
| Dose of carboplatin | 300 mg/m2 | 300 mg/m2 |
| Dose of CYTOXAN® | 600 mg/m2 | 600 mg/m2 |
| Residual tumor <2 cm (number of patients) | 39% (174/447) | 14% (49/342) |
| NCIC | SWOG | |
|---|---|---|
| Carboplatin (number of patients) | 60% (48/80) | 58% (48/83) |
| Cisplatin (number of patients) | 58% (49/85) | 43% (33/76) |
| 95% CI of difference (Carboplatin-Cisplatin) | (−13.9%, 18.6%) | (−2.3%, 31.1%) |
| NCIC | SWOG | |
|---|---|---|
| * 114 PARAPLATIN and 109 Cisplatin patients did not undergo second look surgery in NCIC study. | ||
| 90 PARAPLATIN and 106 Cisplatin patients did not undergo second look surgery in SWOG study. | ||
| Carboplatin (number of patients) | 11% (24/224) | 10% (17/171) |
| Cisplatin (number of patients) | 15% (33/223) | 10% (17/171) |
| 95% CI of difference (Carboplatin-Cisplatin) | (−10.7%, 2.5%) | (−6.9%, 6.9%) |
| NCIC | SWOG | |
|---|---|---|
| * Kaplan-Meier
Estimates Unrelated deaths occurring in the absence of progression were counted as events (progression) in this analysis. |
||
| ** Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis. | ||
| Median | ||
| Carboplatin | 59 weeks | 49 weeks |
| Cisplatin | 61 weeks | 47 weeks |
| 2-year PFS* | ||
| Carboplatin | 31% | 21% |
| Cisplatin | 31% | 21% |
| 95% CI of difference (Carboplatin-Cisplatin) | (−9.3, 8.7) | (−9.0, 9.4) |
| 3-year PFS* | ||
| Carboplatin | 19% | 8% |
| Cisplatin | 23% | 14% |
| 95% CI of difference (Carboplatin-Cisplatin) | (−11.5, 4.5) | (−14.1, 0.3) |
| Hazard Ratio** | 1.10 | 1.02 |
| 95% CI (Carboplatin-Cisplatin) | (0.89, 1.35) | (0.81, 1.29) |
| NCIC | SWOG | |
|---|---|---|
| * Kaplan-Meier Estimates | ||
| ** Analysis adjusted for factors found to be of prognostic significance were consistent with unadjusted analysis. | ||
| Median | ||
| Carboplatin | 110 weeks | 86 weeks |
| Cisplatin | 99 weeks | 79 weeks |
| 2-year Survival* | ||
| Carboplatin | 51.9% | 40.2% |
| Cisplatin | 48.4% | 39.0% |
| 95% CI of difference (Carboplatin-Cisplatin) | (−6.2, 13.2) | (−9.8, 12.2) |
| 3-year Survival* | ||
| Carboplatin | 34.6% | 18.3% |
| Cisplatin | 33.1% | 24.9% |
| 95% CI of difference (Carboplatin-Cisplatin) | (−7.7, 10.7) | (−15.9, 2.7) |
| Hazard Ratio** | 0.98 | 1.01 |
| 95% CI (Carboplatin-Cisplatin) | (0.78, 1.23) | (0.78, 1.30) |
Comparative Toxicity
The pattern of toxicity exerted by the PARAPLATIN-containing regimen was significantly different from that of the cisplatin-containing combinations. Differences between the two studies may be explained by different cisplatin dosages and by different supportive care.
The PARAPLATIN-containing regimen induced significantly more thrombocytopenia and, in one study, significantly more leukopenia and more need for transfusional support. The cisplatin-containing regimen produced significantly more anemia in one study. However, no significant differences occurred in incidences of infections and hemorrhagic episodes.
Non-hematologic toxicities (emesis, neurotoxicity, ototoxicity, renal toxicity, hypomagnesemia, and alopecia) were significantly more frequent in the cisplatin-containing arms.
| PARAPLATIN Arm Percent* | Cisplatin Arm Percent* |
P-Values** |
||
|---|---|---|---|---|
| * Values are in percent of evaluable patients. | ||||
| ** ns=not significant, p>0.05. | ||||
| + May have been affected by cyclophosphamide dosage delivered. | ||||
| Bone Marrow | ||||
| Thrombocytopenia | <100,000/mm3 | 70 | 29 | <0.001 |
| <50,000/mm3 | 41 | 6 | <0.001 | |
| Neutropenia | <2000 cells/mm3 | 97 | 96 | ns |
| <1000 cells/mm3 | 81 | 79 | ns | |
| Leukopenia | <4000 cells/mm3 | 98 | 97 | ns |
| <2000 cells/mm3 | 68 | 52 | 0.001 | |
| Anemia | <11 g/dL | 91 | 91 | ns |
| <8 g/dL | 18 | 12 | ns | |
| Infections | 14 | 12 | ns | |
| Bleeding | 10 | 4 | ns | |
| Transfusions | 42 | 31 | 0.018 | |
| Gastrointestinal | ||||
| Nausea and vomiting | 93 | 98 | 0.010 | |
| Vomiting | 84 | 97 | <0.001 | |
| Other GI side effects | 50 | 62 | 0.013 | |
| Neurologic | ||||
| Peripheral neuropathies | 16 | 42 | <0.001 | |
| Ototoxicity | 13 | 33 | <0.001 | |
| Other sensory side effects | 6 | 10 | ns | |
| Central neurotoxicity | 28 | 40 | 0.009 | |
| Renal | ||||
| Serum creatinine elevations | 5 | 13 | 0.006 | |
| Blood urea elevations | 17 | 31 | <0.001 | |
| Hepatic | ||||
| Bilirubin elevations | 5 | 3 | ns | |
| SGOT elevations | 17 | 13 | ns | |
| Alkaline phosphatase elevations | — | — | — | |
| Electrolytes loss | ||||
| Sodium | 10 | 20 | 0.005 | |
| Potassium | 16 | 22 | ns | |
| Calcium | 16 | 19 | ns | |
| Magnesium | 63 | 88 | <0.001 | |
| Other side effects | ||||
| Pain | 36 | 37 | ns | |
| Asthenia | 40 | 33 | ns | |
| Cardiovascular | 15 | 19 | ns | |
| Respiratory | 8 | 9 | ns | |
| Allergic | 12 | 9 | ns | |
| Genitourinary | 10 | 10 | ns | |
| Alopecia + | 50 | 62 | 0.017 | |
| Mucositis | 10 | 9 | ns | |
| PARAPLATIN Arm Percent* | Cisplatin Arm Percent* |
P-Values** |
||
|---|---|---|---|---|
| * Values are in percent of evaluable patients. | ||||
| ** ns=not significant, p >0.05. | ||||
| + May have been affected by cyclophosphamide dosage delivered. | ||||
| Bone Marrow | ||||
| Thrombocytopenia | <100,000/mm3 | 59 | 35 | <0.001 |
| <50,000/mm3 | 22 | 11 | 0.006 | |
| Neutropenia | <2000 cells/mm3 | 95 | 97 | ns |
| <1000 cells/mm3 | 84 | 78 | ns | |
| Leukopenia | <4000 cells/mm3 | 97 | 97 | ns |
| <2000 cells/mm3 | 76 | 67 | ns | |
| Anemia | <11 g/dL | 88 | 87 | ns |
| <8 g/dL | 8 | 24 | <0.001 | |
| Infections | 18 | 21 | ns | |
| Bleeding | 6 | 4 | ns | |
| Transfusions | 25 | 33 | ns | |
| Gastrointestinal | ||||
| Nausea and vomiting | 94 | 96 | ns | |
| Vomiting | 82 | 91 | 0.007 | |
| Other GI side effects | 40 | 48 | ns | |
| Neurologic | ||||
| Peripheral neuropathies | 13 | 28 | 0.001 | |
| Ototoxicity | 12 | 30 | <0.001 | |
| Other sensory side effects | 4 | 6 | ns | |
| Central neurotoxicity | 23 | 29 | ns | |
| Renal | ||||
| Serum creatinine elevations | 7 | 38 | <0.001 | |
| Blood urea elevations | — | — | — | |
| Hepatic | ||||
| Bilirubin elevations | 5 | 3 | ns | |
| SGOT elevations | 23 | 16 | ns | |
| Alkaline phosphatase elevations | 29 | 20 | ns | |
| Electrolytes loss | ||||
| Sodium | — | — | — | |
| Potassium | — | — | — | |
| Calcium | — | — | — | |
| Magnesium | 58 | 77 | <0.001 | |
| Other side effects | ||||
| Pain | 54 | 52 | ns | |
| Asthenia | 43 | 46 | ns | |
| Cardiovascular | 23 | 30 | ns | |
| Respiratory | 12 | 11 | ns | |
| Allergic | 10 | 11 | ns | |
| Genitourinary | 11 | 13 | ns | |
| Alopecia + | 43 | 57 | 0.009 | |
| Mucositis | 6 | 11 | ns | |
Use as a Single Agent for Secondary Treatment of Advanced Ovarian Cancer
In two prospective, randomized controlled studies in patients with advanced ovarian cancer previously treated with chemotherapy, PARAPLATIN (carboplatin) for Injection, USP achieved six clinical complete responses in 47 patients. The duration of these responses ranged from 45 to 71+ weeks.
INDICATIONS
Initial Treatment of Advanced Ovarian Carcinoma
PARAPLATIN (carboplatin) for Injection, USP is indicated for the initial treatment of advanced ovarian carcinoma in established combination with other approved chemotherapeutic agents. One established combination regimen consists of PARAPLATIN and cyclophosphamide. Two randomized controlled studies conducted by the NCIC and SWOG with PARAPLATIN versus cisplatin, both in combination with cyclophosphamide, have demonstrated equivalent overall survival between the two groups (see CLINICAL STUDIES).
There is limited statistical power to demonstrate equivalence in overall pathologic complete response rates and long-term survival (≥3 years) because of the small number of patients with these outcomes: the small number of patients with residual tumor <2 cm after initial surgery also limits the statistical power to demonstrate equivalence in this subgroup.
Secondary Treatment of Advanced Ovarian Carcinoma
PARAPLATIN is indicated for the palliative treatment of patients with ovarian carcinoma recurrent after prior chemotherapy, including patients who have been previously treated with cisplatin.
Within the group of patients previously treated with cisplatin, those who have developed progressive disease while receiving cisplatin therapy may have a decreased response rate.
CONTRAINDICATIONS
PARAPLATIN is contraindicated in patients with a history of severe allergic reactions to cisplatin or other platinum-containing compounds, or mannitol.
PARAPLATIN (carboplatin) for Injection, USP should not be employed in patients with severe bone marrow depression or significant bleeding.
WARNINGS
Bone marrow suppression (leukopenia, neutropenia, and thrombocytopenia) is dose-dependent and is also the dose-limiting toxicity. Peripheral blood counts should be frequently monitored during PARAPLATIN treatment and, when appropriate, until recovery is achieved. Median nadir occurs at day 21 in patients receiving single-agent PARAPLATIN. In general, single intermittent courses of PARAPLATIN should not be repeated until leukocyte, neutrophil, and platelet counts have recovered.
Since anemia is cumulative, transfusions may be needed during treatment with PARAPLATIN, particularly in patients receiving prolonged therapy.
Bone marrow suppression is increased in patients who have received prior therapy, especially regimens including cisplatin. Marrow suppression is also increased in patients with impaired kidney function. Initial PARAPLATIN dosages in these patients should be appropriately reduced (see DOSAGE AND ADMINISTRATION) and blood counts should be carefully monitored between courses. The use of PARAPLATIN in combination with other bone marrow suppressing therapies must be carefully managed with respect to dosage and timing in order to minimize additive effects.
PARAPLATIN has limited nephrotoxic potential, but concomitant treatment with aminoglycosides has resulted in increased renal and/or audiologic toxicity, and caution must be exercised when a patient receives both drugs. Clinically significant hearing loss has been reported to occur in pediatric patients when PARAPLATIN was administered at higher than recommended doses in combination with other ototoxic agents.
PARAPLATIN can induce emesis, which can be more severe in patients previously receiving emetogenic therapy. The incidence and intensity of emesis have been reduced by using premedication with antiemetics. Although no conclusive efficacy data exist with the following schedules of PARAPLATIN, lengthening the duration of single intravenous administration to 24 hours or dividing the total dose over 5 consecutive daily pulse doses has resulted in reduced emesis.
Although peripheral neurotoxicity is infrequent, its incidence is increased in patients older than 65 years and in patients previously treated with cisplatin. Pre-existing cisplatin-induced neurotoxicity does not worsen in about 70% of the patients receiving PARAPLATIN as secondary treatment.
Loss of vision, which can be complete for light and colors, has been reported after the use of PARAPLATIN with doses higher than those recommended in the package insert. Vision appears to recover totally or to a significant extent within weeks of stopping these high doses.
As in the case of other platinum-coordination compounds, allergic reactions to PARAPLATIN have been reported. These may occur within minutes of administration and should be managed with appropriate supportive therapy. There is increased risk of allergic reactions including anaphylaxis in patients previously exposed to platinum therapy. (See CONTRAINDICATIONS and ADVERSE REACTIONS: Allergic Reactions.)
High dosages of PARAPLATIN (more than 4 times the recommended dose) have resulted in severe abnormalities of liver function tests.
PARAPLATIN may cause fetal harm when administered to a pregnant woman. PARAPLATIN has been shown to be embryotoxic and teratogenic in rats. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General
Needles or intravenous administration sets containing aluminum parts that may come in contact with PARAPLATIN should not be used for the preparation or administration of the drug. Aluminum can react with carboplatin causing precipitate formation and loss of potency.
Drug Interactions
The renal effects of nephrotoxic compounds may be potentiated by PARAPLATIN.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of carboplatin has not been studied, but compounds with similar mechanisms of action and mutagenicity profiles have been reported to be carcinogenic. Carboplatin has been shown to be mutagenic both in vitro and in vivo. It has also been shown to be embryotoxic and teratogenic in rats receiving the drug during organogenesis. Secondary malignancies have been reported in association with multi-drug therapy.
Pregnancy
Pregnancy Category D
See WARNINGS.
Nursing Mothers
It is not known whether carboplatin is excreted in human milk. Because there is a possibility of toxicity in nursing infants secondary to PARAPLATIN treatment of the mother, it is recommended that breast feeding be discontinued if the mother is treated with PARAPLATIN (carboplatin) for Injection, USP.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established (see WARNINGS; “audiologic toxicity”).
Geriatric Use
Of the 789 patients in initial treatment combination therapy studies (NCIC and SWOG), 395 patients were treated with carboplatin in combination with cyclophosphamide. Of these, 141 were over 65 years of age and 22 were 75 years or older. In these trials, age was not a prognostic factor for survival. In terms of safety, elderly patients treated with carboplatin were more likely to develop severe thrombocytopenia than younger patients. In a combined database of 1,942 patients (414 were ≥65 years of age) that received single-agent carboplatin for different tumor types, a similar incidence of adverse events was seen in patients 65 years and older and in patients less than 65. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Because renal function is often decreased in the elderly, renal function should be considered in the selection of PARAPLATIN dosage (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
For a comparison of toxicities when carboplatin or cisplatin was given in combination with cyclophosphamide, see CLINICAL STUDIES: Comparative Toxicity.
| First Line Combination Therapy* Percent | Second Line Single Agent Therapy** Percent |
||
|---|---|---|---|
| * Use with Cyclophosphamide for Initial Treatment of Ovarian Cancer: Data are based on the experience of 393 patients with ovarian cancer (regardless of baseline status) who received initial combination therapy with PARAPLATIN and cyclophosphamide in two randomized controlled studies conducted by SWOG and NCIC (see CLINICAL STUDIES). | |||
| Combination with cyclophosphamide as well as duration of treatment may be responsible for the differences that can be noted in the adverse experience table. | |||
| **Single Agent Use for the Secondary Treatment of Ovarian Cancer: Data are based on the experience of 553 patients with previously treated ovarian carcinoma (regardless of baseline status) who received single-agent PARAPLATIN. | |||
| Bone Marrow | |||
| Thrombocytopenia | <100,000/mm3 | 66 | 62 |
| <50,000/mm3 | 33 | 35 | |
| Neutropenia | <2000 cells/mm3 | 96 | 67 |
| <1000 cells/mm3 | 82 | 21 | |
| Leukopenia | <4000 cells/mm3 | 97 | 85 |
| <2000 cells/mm3 | 71 | 26 | |
| Anemia | <11 g/dL | 90 | 90 |
| <8 g/dL | 14 | 21 | |
| Infections | 16 | 5 | |
| Bleeding | 8 | 5 | |
| Transfusions | 35 | 44 | |
| Gastrointestinal | |||
| Nausea and vomiting | 93 | 92 | |
| Vomiting | 83 | 81 | |
| Other GI side effects | 46 | 21 | |
| Neurologic | |||
| Peripheral neuropathies | 15 | 6 | |
| Ototoxicity | 12 | 1 | |
| Other sensory side effects | 5 | 1 | |
| Central neurotoxicity | 26 | 5 | |
| Renal | |||
| Serum creatinine elevations | 6 | 10 | |
| Blood urea elevations | 17 | 22 | |
| Hepatic | |||
| Bilirubin elevations | 5 | 5 | |
| SGOT elevations | 20 | 19 | |
| Alkaline phosphatase elevations | 29 | 37 | |
| Electrolytes loss | |||
| Sodium | 10 | 47 | |
| Potassium | 16 | 28 | |
| Calcium | 16 | 31 | |
| Magnesium | 61 | 43 | |
| Other side effects | |||
| Pain | 44 | 23 | |
| Asthenia | 41 | 11 | |
| Cardiovascular | 19 | 6 | |
| Respiratory | 10 | 6 | |
| Allergic | 11 | 2 | |
| Genitourinary | 10 | 2 | |
| Alopecia | 49 | 2 | |
| Mucositis | 8 | 1 | |
In the narrative section that follows, the incidences of adverse events are based on data from 1,893 patients with various types of tumors who received PARAPLATIN as single-agent therapy.
Hematologic Toxicity
Bone marrow suppression is the dose-limiting toxicity of PARAPLATIN. Thrombocytopenia with platelet counts below 50,000/mm3 occurs in 25% of the patients (35% of pretreated ovarian cancer patients); neutropenia with granulocyte counts below 1,000/mm3 occurs in 16% of the patients (21% of pretreated ovarian cancer patients); leukopenia with WBC counts below 2,000/mm3 occurs in 15% of the patients (26% of pretreated ovarian cancer patients). The nadir usually occurs about day 21 in patients receiving single-agent therapy. By day 28, 90% of patients have platelet counts above 100,000/mm3; 74% have neutrophil counts above 2,000/mm3; 67% have leukocyte counts above 4,000/mm3.
Marrow suppression is usually more severe in patients with impaired kidney function. Patients with poor performance status have also experienced a higher incidence of severe leukopenia and thrombocytopenia.
The hematologic effects, although usually reversible, have resulted in infectious or hemorrhagic complications in 5% of the patients treated with PARAPLATIN, with drug-related death occurring in less than 1% of the patients. Fever has also been reported in patients with neutropenia.
Anemia with hemoglobin less than 11 g/dL has been observed in 71% of the patients who started therapy with a baseline above that value. The incidence of anemia increases with increasing exposure to PARAPLATIN. Transfusions have been administered to 26% of the patients treated with PARAPLATIN (44% of previously treated ovarian cancer patients).
Bone marrow depression may be more severe when PARAPLATIN is combined with other bone marrow suppressing drugs or with radiotherapy.
Gastrointestinal Toxicity
Vomiting occurs in 65% of the patients (81% of previously treated ovarian cancer patients) and in about one-third of these patients it is severe. Carboplatin, as a single agent or in combination, is significantly less emetogenic than cisplatin; however, patients previously treated with emetogenic agents, especially cisplatin, appear to be more prone to vomiting. Nausea alone occurs in an additional 10% to 15% of patients. Both nausea and vomiting usually cease within 24 hours of treatment and are often responsive to antiemetic measures. Although no conclusive efficacy data exist with the following schedules, prolonged administration of PARAPLATIN, either by continuous 24-hour infusion or by daily pulse doses given for 5 consecutive days, was associated with less severe vomiting than the single-dose intermittent schedule. Emesis was increased when PARAPLATIN was used in combination with other emetogenic compounds. Other gastrointestinal effects observed frequently were pain, in 17% of the patients; diarrhea, in 6%; and constipation, also in 6%.
Neurologic Toxicity
Peripheral neuropathies have been observed in 4% of the patients receiving PARAPLATIN (6% of pretreated ovarian cancer patients) with mild paresthesias occurring most frequently. Carboplatin therapy produces significantly fewer and less severe neurologic side effects than does therapy with cisplatin. However, patients older than 65 years and/or previously treated with cisplatin appear to have an increased risk (10%) for peripheral neuropathies. In 70% of the patients with pre-existing cisplatin-induced peripheral neurotoxicity, there was no worsening of symptoms during therapy with PARAPLATIN. Clinical ototoxicity and other sensory abnormalities such as visual disturbances and change in taste have been reported in only 1% of the patients. Central nervous system symptoms have been reported in 5% of the patients and appear to be most often related to the use of antiemetics.
Although the overall incidence of peripheral neurologic side effects induced by PARAPLATIN is low, prolonged treatment, particularly in cisplatin pretreated patients, may result in cumulative neurotoxicity.
Nephrotoxicity
Development of abnormal renal function test results is uncommon, despite the fact that carboplatin, unlike cisplatin, has usually been administered without high-volume fluid hydration and/or forced diuresis. The incidences of abnormal renal function tests reported are 6% for serum creatinine and 14% for blood urea nitrogen (10% and 22%, respectively, in pretreated ovarian cancer patients). Most of these reported abnormalities have been mild and about one-half of them were reversible.
Creatinine clearance has proven to be the most sensitive measure of kidney function in patients receiving PARAPLATIN, and it appears to be the most useful test for correlating drug clearance and bone marrow suppression. Twenty-seven percent of the patients who had a baseline value of 60 mL/min or more demonstrated a reduction below this value during PARAPLATIN therapy.
Hepatic Toxicity
The incidences of abnormal liver function tests in patients with normal baseline values were reported as follows: total bilirubin, 5%; SGOT, 15%; and alkaline phosphatase, 24%; (5%, 19%, and 37%, respectively, in pretreated ovarian cancer patients). These abnormalities have generally been mild and reversible in about one-half of the cases, although the role of metastatic tumor in the liver may complicate the assessment in many patients. In a limited series of patients receiving very high dosages of PARAPLATIN and autologous bone marrow transplantation, severe abnormalities of liver function tests were reported.
Electrolyte Changes
The incidences of abnormally decreased serum electrolyte values reported were as follows: sodium, 29%; potassium, 20%; calcium, 22%; and magnesium, 29%; (47%, 28%, 31%, and 43%, respectively, in pretreated ovarian cancer patients). Electrolyte supplementation was not routinely administered concomitantly with PARAPLATIN, and these electrolyte abnormalities were rarely associated with symptoms.
Allergic Reactions
Hypersensitivity to PARAPLATIN has been reported in 2% of the patients. These allergic reactions have been similar in nature and severity to those reported with other platinum-containing compounds, ie, rash, urticaria, erythema, pruritus, and rarely bronchospasm and hypotension. Anaphylactic reactions have been reported as part of postmarketing surveillance (see WARNINGS). These reactions have been successfully managed with standard epinephrine, corticosteroid, and antihistamine therapy.
Injection Site Reactions
Injection site reactions, including redness, swelling, and pain, have been reported during postmarketing surveillance. Necrosis associated with extravasation has also been reported.
Other Events
Pain and asthenia were the most frequently reported miscellaneous adverse effects; their relationship to the tumor and to anemia was likely. Alopecia was reported (3%). Cardiovascular, respiratory, genitourinary, and mucosal side effects have occurred in 6% or less of the patients. Cardiovascular events (cardiac failure, embolism, cerebrovascular accidents) were fatal in less than 1% of the patients and did not appear to be related to chemotherapy. Cancer-associated hemolytic uremic syndrome has been reported rarely.
Malaise, anorexia, hypertension, dehydration, and stomatitis have been reported as part of postmarketing surveillance.
OVERDOSAGE
There is no known antidote for PARAPLATIN overdosage. The anticipated complications of overdosage would be secondary to bone marrow suppression and/or hepatic toxicity.
DOSAGE AND ADMINISTRATION
NOTE: Aluminum reacts with carboplatin causing precipitate formation and loss of potency, therefore, needles or intravenous sets containing aluminum parts that may come in contact with the drug must not be used for the preparation or administration of PARAPLATIN.
Single-Agent Therapy
PARAPLATIN (carboplatin) for Injection, USP, as a single agent, has been shown to be effective in patients with recurrent ovarian carcinoma at a dosage of 360 mg/m2 IV on day 1 every 4 weeks (alternatively see Formula Dosing). In general, however, single intermittent courses of PARAPLATIN should not be repeated until the neutrophil count is at least 2,000 and the platelet count is at least 100,000.
Combination Therapy with Cyclophosphamide
In the chemotherapy of advanced ovarian cancer, an effective combination for previously untreated patients consists of:
PARAPLATIN—300 mg/m2 IV on day 1 every 4 weeks for 6 cycles (alternatively see Formula Dosing).
Cyclophosphamide—600 mg/m2 IV on day 1 every 4 weeks for 6 cycles. For directions regarding the use and administration of cyclophosphamide please refer to its package insert. (See CLINICAL STUDIES.)
Intermittent courses of PARAPLATIN in combination with cyclophosphamide should not be repeated until the neutrophil count is at least 2,000 and the platelet count is at least 100,000.
Dose Adjustment Recommendations
Pretreatment platelet count and performance status are important prognostic factors for severity of myelosuppression in previously treated patients.
The suggested dose adjustments for single agent or combination therapy shown in the table below are modified from controlled trials in previously treated and untreated patients with ovarian carcinoma. Blood counts were done weekly, and the recommendations are based on the lowest post-treatment platelet or neutrophil value.
| *Percentages apply to PARAPLATIN (carboplatin) for Injection, USP as a single agent or to both PARAPLATIN and cyclophosphamide in combination. In the controlled studies, dosages were also adjusted at a lower level (50% to 60%) for severe myelosuppression. Escalations above 125% were not recommended for these studies. | ||
|
Platelets |
Neutrophils | Adjusted Dose* (From Prior Course) |
| >100,000 | >2,000 | 125% |
| 50-100,000 | 500-2,000 | No Adjustment |
| <50,000 | <500 | 75% |
PARAPLATIN is usually administered by an infusion lasting 15 minutes or longer. No pre- or post-treatment hydration or forced diuresis is required.
Patients with Impaired Kidney Function
Patients with creatinine clearance values below 60 mL/min are at increased risk of severe bone marrow suppression. In renally-impaired patients who received single-agent PARAPLATIN therapy, the incidence of severe leukopenia, neutropenia, or thrombocytopenia has been about 25% when the dosage modifications in the table below have been used.
| Baseline Creatinine Clearance | Recommended Dose on Day 1 |
|---|---|
| 41 mL/min - 59 mL/min | 250 mg/m2 |
| 16 mL/min - 40 mL/min | 200 mg/m2 |
The data available for patients with severely impaired kidney function (creatinine clearance below 15 mL/min) are too limited to permit a recommendation for treatment.
These dosing recommendations apply to the initial course of treatment. Subsequent dosages should be adjusted according to the patient’s tolerance based on the degree of bone marrow suppression.
Formula Dosing
Another approach for determining the initial dose of PARAPLATIN is the use of mathematical formulae, which are based on a patient’s pre-existing renal function or renal function and desired platelet nadir. Renal excretion is the major route of elimination for carboplatin. (See CLINICAL PHARMACOLOGY.) The use of dosing formulae, as compared to empirical dose calculation based on body surface area, allows compensation for patient variations in pretreatment renal function that might otherwise result in either underdosing (in patients with above average renal function) or overdosing (in patients with impaired renal function).
A simple formula for calculating dosage, based upon a patient’s glomerular filtration rate (GFR in mL/min) and PARAPLATIN target area under the concentration versus time curve (AUC in mg/mL•min), has been proposed by Calvert. In these studies, GFR was measured by 51Cr-EDTA clearance.
| CALVERT FORMULA FOR CARBOPLATIN DOSING |
| Total Dose (mg)=(target AUC) × (GFR + 25) |
| Note: With the Calvert formula, the total dose of PARAPLATIN is calculated in mg, not mg/m2. |
The target AUC of 4 mg/mL•min to 6 mg/mL•min using single-agent PARAPLATIN appears to provide the most appropriate dose range in previously treated patients. This study also showed a trend between the AUC of single-agent PARAPLATIN administered to previously treated patients and the likelihood of developing toxicity.
| % Actual Toxicity in Previously Treated Patients | ||
|---|---|---|
|
AUC (mg/mL•min) | Gr 3 or Gr 4 Thrombocytopenia | Gr 3 or Gr 4 Leukopenia |
| 4 to 5 | 16% | 13% |
| 6 to 7 | 33% | 34% |
Geriatric Dosing
Because renal function is often decreased in elderly patients, formula dosing of PARAPLATIN based on estimates of GFR should be used in elderly patients to provide predictable plasma PARAPLATIN AUCs and thereby minimize the risk of toxicity.
PREPARATION OF INTRAVENOUS SOLUTIONS
Immediately before use, the content of each vial must be reconstituted with either Sterile Water for Injection, USP, 5% Dextrose in Water (D5W), or 0.9% Sodium Chloride Injection, USP, according to the following schedule:
| Vial Strength | Diluent Volume |
|---|---|
| 50 mg | 5 mL |
| 150 mg | 15 mL |
| 450 mg | 45 mL |
These dilutions all produce a carboplatin concentration of 10 mg/mL.
PARAPLATIN can be further diluted to concentrations as low as 0.5 mg/mL with 5% Dextrose in Water (D5W) or 0.9% Sodium Chloride Injection, USP.
STABILITY
Unopened vials of PARAPLATIN are stable for the life indicated on the package when stored at 25°C (77°F); excursions permitted from 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Protect from light.
When prepared as directed, PARAPLATIN solutions are stable for 8 hours at room temperature (25°C). Since no antibacterial preservative is contained in the formulation, it is recommended that PARAPLATIN solutions be discarded 8 hours after dilution.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
HOW SUPPLIED
PARAPLATIN® (carboplatin) for Injection, USP
NDC 0015-3213-30 50 mg vials, individually
cartoned. (Yellow flip-off seals)
NDC 0015-3214-30 150 mg vials, individually cartoned. (Violet flip-off seals)
NDC 0015-3215-30 450 mg vials,
individually cartoned. (Blue flip-off seals)
Storage
Store the unopened vials at 25°C (77°F); excursions permitted from 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Protect unopened vials from light. Solutions for infusion should be discarded 8 hours after preparation.
Handling and Disposal
Caution should be exercised in handling and preparing PARAPLATIN (carboplatin) for Injection, USP. Several guidelines on this subject have been published.1-4
To minimize the risk of dermal exposure, always wear impervious gloves when handling vials containing PARAPLATIN (carboplatin) for Injection, USP. If PARAPLATIN (carboplatin) for Injection, USP or solutions containing PARAPLATIN (carboplatin) for Injection, USP contact the skin, immediately wash the skin thoroughly with soap and water. If PARAPLATIN (carboplatin) for Injection, USP or solutions containing PARAPLATIN (carboplatin) for Injection, USP contact mucous membranes, the membranes should be flushed immediately and thoroughly with water. More information is available in the references listed below.
REFERENCES
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2004-165.
- OSHA Technical Manual, TED 1-0.15A, Section VI: Chapter 2. Controlling occupational exposure to hazardous drugs. OSHA, 1999. http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html
- American Society of Health-System Pharmacists. ASHP guidelines on handling hazardous drugs. Am J Health-Syst Pharm. 2006;63:1172-1193.
- Polovich M, White JM, Kelleher LO, eds. 2005. Chemotherapy and biotherapy guidelines and recommendations for practice. 2nd ed. Pittsburgh, PA: Oncology Nursing Society.
Bristol-Myers
Squibb Company
Princeton, New Jersey 08543 USA
Made in Italy
Rev July 2010
---------------------------------------------
REPRESENTATIVE PACKAGING
Not Applicable - No Longer Marketed

| PARAPLATIN
carboplatin injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019880 | 01/01/2008 | 03/14/2009 |
| PARAPLATIN
carboplatin injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019880 | 01/01/2008 | 05/14/2009 |
| PARAPLATIN
carboplatin injection, powder, lyophilized, for solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA019880 | 01/01/2008 | 04/14/2009 |
| Labeler - E.R. Squibb & Sons, L.L.C. (006370092) |