EPINEPHRINE
-
epinephrine hydrochloride injection, solution
Claris Lifesciences Inc.
----------
EPINEPHrine Injection, USP1:1000 (1 mg/ml)
1 mL single close glass ampoule
and 30 mL multiple close glass vial Protect from light until ready to use
Rx only
Epinephrine Injection, USP 1:1000 is a sterile, nonpyrogenic solution. When diluted, it may also be administered intracardially or intravenously. Each mL of single dose ampoule contains epinephrine 1 mg; sodium chloride 9 mg; sodium metabisulfite 0.9 mg added. May contain hydrochloric acid for pH adjustment.
Each mL of multiple dose vial contains: 1 mg Epinephrine as the hydrochloride, in Water for Injection, USP, q.s. Sodium Chloride added for isotonicity, 0.5% Chlorobutanol (a chloroform derivative) as a preservative and not more than 0.15% Sodium Metabisulfite as an antioxidant. The pH may be adjusted with Sodium Hydroxide and/ or Hydrochloric Acid.
It is administered by the following routes: intravenous, intracardiac (left ventricular chamber), via endotracheal tube into the bronchial tree, subcutaneous or intramuscular.
Epinephrine, USP is a sympathomimetic (adrenergic) agent designated chemically as (-)-3,4-Dihydroxy-a-[(methylamino)methyl]benzyl alcohol, a white, microcrystalline powder. It has the following structural formula:
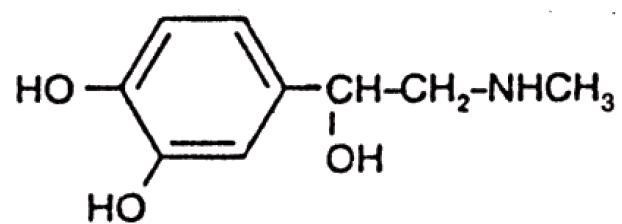
Sodium Chloride, USP is chemically designated NaCI, a white, crystalline compound freely soluble in water.
The actions of epinephrine resemble the effects of stimulation of adrenergic nerves. To a variable degree it acts on both alpha and beta receptor sites of sympathetic effector cells. Its most prominent actions are on the beta-receptors of the heart, vascular and other smooth muscle. When given by rapid intravenous injection, it produces a rapid rise in blood pressure, mainly systolic, by (1) direct stimulation of cardiac muscle which increases the strength of ventricular contraction, (2) increasing the heart rate and (3) constriction of the arterioles in the skin, mucosa and splanchnic areas of the circulation.
When given by slow intravenous injection epinephrine usually produces only a moderate rise in systolic and a fall in diastolic pressure. Although some increase in pulse pressure occurs, there is usually no great elevation in mean blood pressure. Accordingly, the compensatory reflex mechanisms that come into play with a pronounced increase in blood pressure do not antagonize the direct cardiac actions of epinephrine as much as with catecholamines that have a predominant action on alpha receptors.
Total peripheral resistance decreases by action of epinephrine on beta receptors of the skeletal muscle vasculature and blood flow is thereby enhanced. Usually this vasodilator effect of the drug on the circulation predominates so that the modest rise in systolic pressure which follows slow injection or absorption is mainly the result of direct cardiac stimulation and increase in cardiac output. In some instances peripheral resistance is not altered or may even rise owing to a greater ratio of alpha to beta activity in different vascular areas.
Epinephrine relaxes the smooth muscles of the bronchi and iris and is a physiologic antagonist of histamine. The drug also produces an increase in blood sugar and glycogenolysis in the liver.
Pharmacokinetics:
Intravenous injection produces an immediate and intensified response. Following I.V. injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. administered epinephrine has a rapid onset and short duration of action. Subcutaneous administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes.
The drug becomes fixed in the tissues and is rapidly inactivated chiefly by enzymic transformation to metanephrine or normetanephrine, either of which is subsequently conjugated and excreted in the urine in the form of sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4-hydroxy-mandelic acid (vanillylmandelic acid, VMA) which is also detectable in the urine.
Epinephrine is used to relieve respiratory distress due to bronchospasm, to provide rapid relief of hypersensitivity reactions to drugs and other allergens, and to prolong the action of anesthetics. Its cardiac effects may be of use in restoring cardiac rhythm in cardiac arrest due to various causes, but it is not used in cardiac failure or in hemorrhagic, traumatic, or cardiogenic shock. Epinephrine is used as a hemostatic agent. It is also used in treating mucosal congestion of hay fever, rhinitis, and acute sinusitis; to relieve bronchial asthmatic paroxysms; in syncope due to complete heart block or carotid sinus hypersensitivity; for symptomatic relief of serum sickness, urticaria, angioneurotic edema; for resuscitation in cardiac arrest following anesthetic accidents; in simple (open angle) glaucoma; for relaxation of uterine musculature and to inhibit uterine contractions. Epinephrine Injection can be utilized to prolong the action of anesthetics used in local and regional anesthesia. (See CONTRAINDICATIONS).
Epinephrine is contraindicated in patients with known hypersensitivity to sympathomimetic amines, in patients with angle (congestive) glaucoma, and patients in shock (nonanaphylactic). It should not be used in patients anesthetized with agents such as cyclopropane or halothane as these may sensitize the heart to the arrhythmic action of sympathomimetic drugs. Addition of epinephrine to local anesthetics for injection of certain areas (e.g., fingers, toes, ears, etc.) is contraindicated because of danger that vasoconstriction may result in sloughing of tissue.
Except as diluted for admixture with local anesthetics to reduce absorption and prolong action, epinephrine should not ordinarily be used in those cases where vasopressor drugs may be contraindicated, e.g., in thyrotoxicosis, diabetes, in obstetrics when maternal blood pressure is in excess of 130/80 and in hypertension and other cardiovascular disorders.
Administer with caution to elderly people; to those with cardiovascular disease, hypertension, diabetes, or hyperthyroidism; in psychoneurotic individuals; and in pregnancy.
Patients with long-standing bronchial asthma and emphysema who have developed degenerative heart disease should be administered the drug with extreme caution.
Overdosage or inadvertent intravenous injection of epinephrine may cause cerebrovascular hemorrhage resulting from the sharp rise in blood pressure. (See OVERDOSAGE)
Fatalities may also result from pulmonary edema because of the peripheral constriction and cardiac stimulation produced. Rapidly acting vasodilators, such as nitrites, or alpha blocking agents may counteract the marked pressor effects of epinephrine.
Inadvertently induced high arterial blood pressure may result in angina pectoris, aortic rupture or cerebral hemorrhage. Epinephrine may induce potentially serious cardiac arrhythmias in patients not suffering from heart disease and in patients with organic heart disease or who are receiving drugs that sensitize the myocardium.
Parenterally administered epinephrine initially may produce constriction of renal blood vessels and decrease urine formation. Epinephrine Injection, USP is subject to oxidation and should be protected against exposure to light and stored in light-resistant containers. Epinephrine contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic rather than in non-asthmatic people.
Epinephrine is the preferred treatment for serious allergic or other emergency situations even though this product contains sodium metabisulfite, a sulfite that may in other products cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons. The alternatives to using epinephrine in a life-threatening situation may not be satisfactory. The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations.
Cardiovascular effects:
Inadvertently induced high arterial blood pressure may result in angina pectoris (especially when coronary insufficiency is present), or aortic rupture.
Epinephrine may induce potentially serious cardiac arrhythmias in patients not suffering from heart disease and patients with organic heart disease or who are receiving drugs that sensitize the myocardium. With Epinephrine 1:10,000, a paradoxical but transient lowering of blood pressure, bradycardia and apnea may occur immediately after injection.
Cerebrovascular hemorrhage:
Overdosage or inadvertent I.V. injection of epinephrine may cause cerebrovascular hemorrhage resulting from the sharp rise in blood pressure.
Pulmonary edema:
Fatalities may also result from pulmonary edema because of the peripheral
constriction and cardiac stimulation produced.
PRECAUTIONS
General: Epinephrine injection should be protected from exposure to light. Do not remove container from carton until ready to use. The solution should not be used if it is pinkish or darker than slightly yellow or if it contains a precipitate. Do not administer unless solution is clear and container is intact. Discard unused portion.
Epinephrine is readily destroyed by alkalies and oxidizing agents. In the latter category are oxygen, chlorine, bromine, iodine, permanganates, chromates, nitrites, and salts of easily reducible metals, especially iron.
In obstetrics, if vasopressor drugs are used either to correct hypotension or added to the local anesthetic solution, some oxytocic drugs may cause severe persistent hypertension; even rupture of a cerebral blood vessel may occur during the postpartum period.
All vasopressors should be used cautiously in patients taking monoamine oxidase (MAO) inhibitors.
Administration of epinephrine to patients receiving cyclopropane or halogenated hydrocarbon general anesthetics such as halothane which sensitize the myocardium, may induce cardiac arrhythmia. (See CONTRAINDICATIONS.) When encountered, such arrhythmias may respond to administration of a beta-adrenergic blocking drug. Epinephrine also should be used cautiously with other drugs (e.g., digitalis glycosides) that sensitize the myocardium to the actions of sympathomimetic agents.
Diuretic agents may decrease vascular response to pressor drugs such as epinephrine.
Epinephrine may antagonize the neuron blockade produced by guanethidine resulting in decreased antihypertensive effect and requiring increased dosage of the latter.
Pregnancy Category C. Animal reproduction studies have not been conducted with epinephrine. It is also not known whether epinephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Epinephrine should be given to a pregnant woman only if clearly needed.
Labor and Delivery. Parenteral administration of epinephrine if used to support blood pressure during low or other spinal anesthesia for delivery can cause acceleration of fetal heart rate and should not be used in obstetrics when maternal blood pressure exceeds 130/80. (See CONTRAINDICATIONS.)
For single dose
Although epinephrine can produce ventricular fibrillation, its actions in restoring electrical activity in asystole and in enhancing defibrillation of the fibrillating ventricle are well documented. The drug, however, should be used with caution in patients with ventricular fibrillation.
Epinephrine should be used cautiously in patients with hyperthyroidism, hypertension and cardiac arrhythmias. All vasopressors should be used cautiously in patients taking monoamine oxidase (MAO) inhibitors.
Epinephrine should not be administered concomitantly with other sympathomimetic drugs (such as isoproterenol) because of possible additive effects and increased toxicity. Combined effects may induce serious cardiac arrhythmias. They may be administered alternately when the preceding effect of other such drugs has subsided.
For multiple dose
Use of epinephrine with excessive doses of digitalis, mercurial diuretics, or other drugs that sensitize the heart to arrhythmias is not recommended. Anginal pain may be induced when coronary insufficiency is present.
The effects of epinephrine may be potentiated by tricyclic antidepressants certain antihistamines, e.g., diphenhydramine, tripelennamine, d-chlorpheniramine; and sodium I-thyroxine.
Cyclopropane or halogenated hydrocarbon anesthetics such as halothane which sensitize the myocardium administration of a beta-adrenergic blocking drug.
Propranolol administered concomitantly with epinephrine may block the beta-adrenergic effects of epinephrine, causing hypertension.
Usage in Children: Epinephrine should be administered with caution to infants and children. Syncope has occurred following the administration of epinephrine to asthmatic children.
ADVERSE REACTIONS
Transient and minor side effects of anxiety, headache, fear, and palpitations often occur with therapeutic doses, especially in hyperthyroid individuals. Repeated local injections can result in necrosis at sites of injection from vascular constriction. "Epinephrine-fastness" can occur with prolonged use.
Local: Repeated local injections can result in necrosis at sites of injection from vascular constriction.
Systemic: Cerebral hemorrhage; hemiplegia; subarachnoid hemorrhage; anginal pain in patients with angina pectoris; anxiety; restlessness; throbbing headache; tremor; weakness; dizziness; pallor; respiratory difficulty; palpitation; apprehensiveness; sweating; nausea; vomiting. Such reactions are unlikely when epinephrine is diluted to 1:200,000 for injection with local anesthetic agents.
OVERDOSAGE
Symptoms: Erroneous administration of large doses of epinephrine may lead to precordial distress, vomiting, headache, dyspnea, as well as unusually elevated blood pressure.
Treatment:
Most toxic effects can be counteracted by injection of an alpha-adrenergic blocker and a beta-adrenergic blocker. In the event of a sharp rise in blood pressure, rapid acting vasodilators such as the nitrites, or alpha-adrenergic blocking agents can counteract the marked pressor effects. If prolonged hypotension follows, it may be necessary to administer another pressor drug, such as norepinephrine.
If an epinephrine overdose induces pulmonary edema that interferes with respiration, treatment consists of a rapidly acting alpha-adrenergic blocking drug such as phentolamine and/or intermittent positive pressure respiration.
Treatment of cardiac consists of a beta-adrenergic blocking drug such as propranolol.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia; these may be accompanied by potentially fatal cardiac arrhythmias. Ventricular premature contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (pre-fibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia, and occasionally, by atrioventricular
block.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis and kidney failure. Take suitable corrective measures.
DOSAGE AND ADMINISTRATION
Subcutaneously or intramuscularly — 0.2 to 1 mL (mg). Start with a small dose and increase if required.
Note: The subcutaneous is the preferred route of administration. If given intramuscularly, injection into the buttocks should be avoided. For bronchial asthma in pediatric patients, administer 0.01 mg/kg or 0.3 mg/m2 to a maximum of 0.5 mg subcutaneously, repeated every four hours if required.
Hypersensitivity Reactions
For bronchial asthma and certain allergic manifestations, e.g., angioedema, urticaria, serum sickness, anaphylactic shock, uses epinephrine subcutaneously. The adult intravenous dose for hypersensitivity reactions or to relieve bronchospasm usually ranges from 0.1 to 0.25 mg injected slowly.
Neonates may be given a dose of 0.01 mg per kg of body weight; for the infant 0.05 mg is an adequate initial dose and this may be repeated at 20 to 30 minute intervals in the management of asthma attacks.
Cardiac Resuscitation
A dose of 0.5 mL (0.5 mg) diluted to 10 mL with sodium chloride injection can be administered intravenously or intracardially to restore myocardial contractility.
Intracardiac injection should only be administered by personnel well trained in the technique, if there has not been sufficient time to establish an intravenous route.
External cardiac massage should follow intracardial administration to permit the drug to enter coronary circulation. The drug should be used secondarily to unsuccessful attempts with physical or electromechanical methods.
Ophthalmologic Use
(for producing conjunctival decongestion, to control hemorrhage, produce mydriasis and reduce intraocular pressure) - use a concentration of 1:10,000 (0.1 mg/mL) to 1:1000 (1 mg/mL).
Regional Anesthesia
A final concentration of 1:200,000 of epinephrine injection is recommended for infiltration injection, nerve block, caudal or other epidural blocks. From 0.3 to 0.4 mg of epinephrine (0.3 to 0.4 mL of 1:1000 solution) may be mixed with spinal anesthetic agents.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. (See PRECAUTIONS.)
HOW SUPPLIED
Epinephrine Injection, USP 1:1000 (1 mg/mL) is supplied in a 1 mL ampoule single-dose container and 30 mL vial multiple-dose container.
| 1 mL | Single Dose Ampoule | (5 x 1 mL ampoules) | NDC: 36000-023-05 |
| 30 mL | Multiple Dose Vial | (1 x 30 mL Vial) | NDC: 36000-024-01 |
Store at 20° to 25°C (68° to 77°F) (See USP Controlled Room Temperature). Protect from light and freezing.
Enter section text here
Epinephrine Injection, USP 1 mg/1 mL Carton
NDC 36000-023-05
5 Ampoules of 1 mL
Sterile Single Dose Ampoule
Rx Only
EPINEPHrine Injection, USP
1 mg/1 mL
1:1000
For SC or IM use
Note: Subcutaneous is the preferred route of administration
Claris
| EPINEPHRINE
epinephrine injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 10/07/2009 | ||
| Labeler - Claris Lifesciences Inc. (808114537) |
| Registrant - Claris Lifesciences Limited (862172228) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Claris Lifesciences Limited | 918603338 | manufacture, analysis | |