EPANOVA- omega-3-carboxylic acids capsule, gelatin coated
AstraZeneca Pharmaceuticals LP
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EPANOVA® safely and effectively. See full prescribing information for EPANOVA.
EPANOVA® (omega-3-carboxylic acids) capsules, for oral use Initial U.S. Approval: 2014 INDICATIONS AND USAGEEPANOVA, a fish oil-derived mixture of free fatty acids primarily composed of EPA and DHA, is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥ 3% and greater than placebo) are diarrhea, nausea, abdominal pain or discomfort, and eructation. (6) To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
EPANOVA® (omega-3-carboxylic acids) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
Usage Considerations: Patients should be placed on an appropriate lipid-lowering diet before receiving EPANOVA and should continue this diet during treatment with EPANOVA.
Laboratory studies should be done to ascertain that the triglyceride levels are consistently abnormal before instituting EPANOVA therapy. Attempts should be made to control serum lipids with appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that are contributing to the lipid abnormalities. Medications known to exacerbate hypertriglyceridemia (such as beta blockers, thiazides, estrogens) should be discontinued or changed if possible prior to consideration of triglyceride-lowering drug therapy.
Limitations of Use:
The effect of EPANOVA on the risk for pancreatitis has not been determined.
The effect of EPANOVA on cardiovascular mortality and morbidity has not been determined.
2 DOSAGE AND ADMINISTRATION
The dosage of EPANOVA is 2 grams (2 capsules) or 4 grams (4 capsules) once daily. The dosage should be individualized according to the patient’s response and tolerability. In clinical trials, EPANOVA was administered without regard to meals.
Patients should be advised to swallow EPANOVA capsules whole. Do not break open, crush, dissolve or chew EPANOVA.
3 DOSAGE FORMS AND STRENGTHS
EPANOVA (omega-3-carboxylic acids) capsules are supplied as 1-gram, red/brown coated, soft-gelatin capsules imprinted with OME1.
4 CONTRAINDICATIONS
EPANOVA is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to EPANOVA or any of its components.
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring: Laboratory Tests
In some patients, EPANOVA increases LDL-C levels. LDL-C levels should be monitored periodically during therapy with EPANOVA.
In patients with hepatic impairment, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels should be monitored periodically during therapy with EPANOVA.
5.2 Fish Allergy
EPANOVA contains polyunsaturated free fatty acids derived from fish oils. It is not known whether patients with allergies to fish and/or shellfish, are at increased risk of an allergic reaction to EPANOVA. EPANOVA should be used with caution in patients with known hypersensitivity to fish and/or shellfish.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported by at least 3% of EPANOVA-treated individuals and with a higher incidence than placebo (olive oil), based on pooled data from two clinical trials of 6- and 12-week duration involving subjects with hypertriglyceridemia, are listed in Table 1.
| * Trials included subjects with hypertriglyceridemia of varying severity. | |||
|
Adverse Reaction |
Placebo
|
EPANOVA 2g
|
EPANOVA 4g
|
|
Diarrhea |
2% |
7% |
15% |
|
Nausea |
1% |
4% |
6% |
|
Abdominal pain or discomfort |
2% |
3% |
5% |
|
Eructation |
<1% |
3% |
3% |
In a pool of two longer-term (≥52 weeks) placebo-controlled clinical trials involving 748 patients (376 EPANOVA 4 grams per day; 372 placebo) with chronic gastrointestinal disease, additional common adverse reactions reported more often by EPANOVA-treated patients included abdominal distension, constipation, vomiting, fatigue, nasopharyngitis, arthralgia, and dysguesia.
7 DRUG INTERACTIONS
7.1 Anticoagulants or Other Drugs Affecting Coagulation
Patients taking anti-platelet agents or anticoagulants were excluded from EPANOVA clinical trials involving patients with hypertriglyceridemia. Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. The prolongation of bleeding time reported in those studies has not exceeded normal limits and did not produce clinically significant bleeding episodes. Nonetheless, patients receiving treatment with EPANOVA and drugs affecting coagulation (e.g., anti-platelet agents) should be monitored periodically.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. It is unknown whether EPANOVA can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. EPANOVA should be used during pregnancy only if the potential benefit to the patient justifies the potential risk to the fetus.
In female rats given oral gavage doses of 100, 600, and 2,000 mg/kg/day beginning 2 weeks prior to mating and continuing through day 6 of gestation, no adverse effects were observed in the high-dose group (5 times human systemic exposure following an oral dose of 4 grams/day based on body surface area comparison).
In pregnant rats given oral gavage doses of 100, 600, and 2,000 mg/kg/day from gestation day 6 through organogenesis , late embryonic deaths and embryos with skeletal variations were observed (5 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
In pregnant rabbits given oral gavage doses of 100, 500, and 750 mg/kg/day from gestation day 6 through organogenesis, skeletal malformations, variations in ossification, and visceral variations were observed in the fetuses in groups given up to 500 mg/kg/day (2 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison). At 750 mg/kg/day, several rabbits aborted and evidence of maternal toxicity was observed, and there was an increase in the incidence of fetuses with malformations and variations (4 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
In a multigenerational developmental study in pregnant rats given oral gavage doses of 100, 600, and 2,000 mg/kg/day from gestation day 6 through lactation day 21, difficulties during and shortly after parturition led to morbidity/mortality in 9 of 24 dams given the highest dose (5 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison). There were no abnormalities observed in offspring (F1) from treated dams. However, survival was decreased from day 10 of lactation onward in second generation offspring (F2) from dams given 600 mg/kg/day (1.5 times the human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
8.2 Labor and Delivery
There are no human studies that have investigated the effects of EPANOVA on preterm labor or labor at term. However, animal studies showed that omega-3 fatty acids caused delayed parturition and associated fetal death in rats (5 times the human systemic exposure following an oral dose of 4 g/day based on body surface area comparison), and premature birth and abortion in rabbits (4 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
8.3 Nursing Mothers
Studies with omega-3 fatty acids derived from fish oil have demonstrated excretion in human milk at levels higher than that in plasma. The effect of this excretion on the infant of a nursing mother is unknown; caution should be exercised when EPANOVA is administered to a nursing mother.
8.5 Geriatric Use
Clinical studies of EPANOVA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
11 DESCRIPTION
EPANOVA, a lipid-regulating agent, is a coated soft-gelatin capsule containing 1 gram of fish oil-derived free fatty acids, designated “omega-3-carboxylic acids,” with at least 850 mg of polyunsaturated fatty acids, including multiple omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA] being the most abundant).
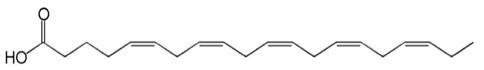
The empirical formula of EPA free fatty acid is C20H30O2, and the molecular weight of EPA free fatty acid is 302.45. The structural formula of EPA free fatty acid is:
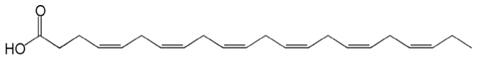
The empirical formula of DHA free fatty acid is C22H32O2, and the molecular weight of DHA free fatty acid is 328.49. The structural formula of DHA free fatty acid is:
EPANOVA capsules also contain the following inactive ingredients: 3 mg α-tocopherol (in a carrier of vegetable oil), and porcine Type A gelatin, glycerol, sorbitol, and purified water (components of the capsule shell). Coating and ink components to the EPANOVA capsules also contain ethyl acrylate and methyl methacrylate copolymer dispersion, talc, titanium dioxide, iron oxide red, polysorbate 80, and carboxymethylcellulose sodium (coating components), pharmaceutical glaze, titanium dioxide, n-butyl alcohol, propylene glycol and isopropanol (ink components).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of EPANOVA is not completely understood. Potential mechanisms of action include inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase, increased mitochondrial and peroxisomal β-oxidation in the liver, decreased lipogenesis in the liver, and increased plasma lipoprotein lipase activity. EPANOVA may reduce the synthesis of triglycerides in the liver because EPA and DHA are poor substrates for the enzymes responsible for TG synthesis, and EPA and DHA inhibit esterification of other fatty acids.
12.3 Pharmacokinetics
Absorption: After oral administration, EPANOVA is directly absorbed in the small intestine, subsequently entering the systemic circulation mainly via the thoracic duct lymphatic system. Following repeat dosing with EPANOVA 4 grams per day under low-fat meal conditions for approximately 2 weeks, maximum plasma concentrations are achieved between 5-8 hours after dosing for total EPA and between 5-9 hours after dosing for total DHA. Steady-state concentrations of EPA and DHA in plasma are achieved within 2 weeks of repeat daily dosing with EPANOVA.
Single-dose administration of EPANOVA with a high-fat meal resulted in an increase in overall exposure of total and free baseline-adjusted EPA by approximately 140% and 80%, respectively, compared to fasting conditions. There was no change in overall exposure of baseline-adjusted total DHA; however, there was a 40% increase in AUC for baseline-adjusted free DHA. Overall exposures of unadjusted total and free EPA increased by 80% and 50%, respectively, although there was no change in overall exposure for unadjusted total and free DHA.
EPANOVA was administered without regard to meals in all clinical trials.
Distribution: Following a single 4-gram dose of EPANOVA under fasted conditions, the vast majority of EPA and DHA in plasma is incorporated in phospholipids, triglycerides and cholesteryl esters, with the free unesterified fatty acid representing approximately 0.8% and 1.1% of the total measured amount for EPA and DHA, respectively.
Metabolism and Excretion: EPA and DHA from EPANOVA are mainly oxidized in the liver similar to fatty acids derived from dietary sources. Following repeat dosing under low-fat meal conditions, the total apparent plasma clearance (CL/F) and half-life of baseline-adjusted EPA from EPANOVA at steady-state are 548 mL/hr and 37 hours, respectively. Under the same conditions, the CL/F and half-life of baseline-adjusted DHA are 518 mL/hr and approximately 46 hours, respectively. EPANOVA does not undergo renal excretion.
Specific Populations
Pediatric: Pharmacokinetics of EPANOVA in pediatric patients have not been studied [see Use in Specific Populations (8.4)].
Renal or Hepatic Impairment: EPANOVA has not been studied in patients with renal or hepatic impairment.
Drug-Drug Interactions
Simvastatin: In a 14-day study of 52 healthy adult subjects, daily co-administration of simvastatin 40 mg with EPANOVA 4 grams did not affect the extent (AUC) or rate (Cmax) of exposure to simvastatin or its major active metabolite, beta-hydroxy simvastatin, at steady state.
Warfarin: In a 14-day study of 52 healthy adult subjects, EPANOVA 4 grams/day at steady-state did not significantly change the single dose AUC or Cmax of R- and S- warfarin or the anti-coagulation pharmacodynamics of 25 mg warfarin.
In vitro studies of cytochrome P450 inhibition with EPANOVA indicated that EPANOVA administration at clinically relevant doses should not result in inhibition of CYP450 enzymes. In vitro, EPANOVA did not affect multidrug resistance associated protein (MRP) or breast cancer resistance protein (BCRP) transporters.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a Sprague-Dawley rat carcinogenicity study with oral gavage doses of 100, 600, and 2,000 mg/kg/day omega-3 carboxylic acid, males were treated for 84 to 95 weeks without an increased incidence of tumors. In female rats treated for 66 to 95 weeks at 2000 mg/kg/day, an increased incidence of benign ovarian sex cord stromal tumors were observed (up to 5 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison). In a 6-month carcinogenicity study, Tg.rasH2 transgenic mice were treated with oral gavage doses of 500, 1000, 2000, and 4000 mg/kg/day omega-3 carboxylic acid without any increase in the incidence of tumors.
EPANOVA was not mutagenic or clastogenic with or without metabolic activation in the bacterial mutagenesis (Ames) test with Salmonella typhimurium and Escherichia coli or in the chromosomal aberration assay in Chinese hamster ovary cells. EPANOVA was negative in the in vivo rat bone marrow micronucleus assay.
In a rat fertility study with oral gavage doses of 100, 600, and 2,000 mg/kg/day, males were treated from 4 weeks prior to mating, and females were treated for 2 weeks prior to and throughout mating until day 6 of gestation. No adverse effect on male or female fertility was observed at 2,000 mg/kg/day (5 times human systemic exposure following an oral dose of 4 grams/day based on a body surface area comparison).
14 CLINICAL STUDIES
14.1 Severe Hypertriglyceridemia
The effects of EPANOVA in severe hypertriglyceridemia were assessed in a 12-week randomized, placebo (olive oil)-controlled, double-blind, parallel-group trial. After a wash-out period of lipid-altering medications other than statins and ezetimibe, patients whose TG levels were between 500 and 2,000 mg/dL were randomly assigned to placebo or EPANOVA 2, 3, or 4 grams per day. Overall, the median baseline triglyceride level was 694 mg/dL. Median baseline non-HDL-C, LDL-C, and HDL-C levels were 217 mg/dL, 81 mg/dL, and 28 mg/dL, respectively. The study population was mostly Caucasian (92%) and male (77%). The mean age was 52 years and the mean BMI was 31 kg/m2. Thirty-seven percent of patients had diabetes, 35% were treated with a statin and/or ezetimibe, and 29% had baseline TG > 885 mg/dL.
Treatment with EPANOVA led to statistically significant reductions in fasting TG levels (Table 2). Treatment with EPANOVA also resulted in statistically significant reductions in non-HDL-C levels compared with placebo, but increased LDL-C levels (Table 2).
| a Placebo = Olive Oil b Difference = Median of [EPANOVA % Change – Placebo % Change] (Hodges-Lehmann Estimate) c 95% confidence interval of the treatment difference was (‑26%, ‑6%) for EPANOVA 2 g vs. Placebo. d 95% confidence interval of the treatment difference was (‑31%, ‑11%) for EPANOVA 4 g vs. Placebo. † not significant; * for p < 0.05; ** for p < 0.01; *** for p < 0.001 Testing for statistical significance, with multiplicity adjustment where appropriate, was performed for TG, non‑HDL‑C, and HDL‑C. P values were obtained from an ANCOVA model using rank-transformed data that included terms for treatment and use of lipid-altering drugs as factors and the baseline value as a covariate. Testing for statistical significance was not performed for TC, VLDL‑C, LDL‑C, or Apo B. Note: The results from the 3 gram arm were not meaningfully different than the 2 gram arm and are therefore not described. |
||||||||
|
Parameter
|
EPANOVA 2 g
|
EPANOVA 4 g
|
Placeboa
|
EPANOVA 2 g
|
EPANOVA 4 g
|
|||
|
BL |
% Change |
BL |
% Change |
BL |
% Change |
Treatment Difference
|
||
|
TG |
717 |
-25 |
655 |
-31 |
682 |
-10 |
-16**c |
-21***d |
|
Non-HDL-C |
205 |
-8 |
225 |
-8 |
215 |
-1 |
-7* |
-10** |
|
HDL-C |
27 |
+7 |
29 |
+5 |
29 |
+2 |
+5† |
+4† |
|
TC |
241 |
-6 |
254 |
-6 |
246 |
0 |
-6 |
-9 |
|
VLDL-C |
123 |
-25 |
126 |
-35 |
125 |
-11 |
-14 |
-21 |
|
LDL-C |
77 |
+21 |
90 |
+26 |
78 |
+10 |
+13 |
+15 |
|
Apo B |
114 |
+6 |
118 |
+6 |
110 |
+2 |
+3 |
+2 |
The effect of EPANOVA on the risk for pancreatitis has not been determined.
The effect of EPANOVA on cardiovascular mortality and morbidity has not been determined.
16 HOW SUPPLIED/STORAGE AND HANDLING
EPANOVA is supplied as a 1-gram, red/brown, polyacrylate-coated, soft-gelatin capsule bearing the designation OME1. Available in bottles of 60 capsules (NDC 0310-2222-60). Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Do not freeze. Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
EPANOVA should be used with caution in patients with known sensitivity or allergy to fish and/or shellfish [see Warnings and Precautions (5.2)].
Patients should be advised that use of lipid-regulating agents does not reduce the importance of adhering to diet [see Dosage and Administration (2)].
Patients should be advised not to alter EPANOVA capsules in any way and to ingest intact capsules only [see Dosage and Administration (2)].
Instruct patients to take EPANOVA as prescribed. If a dose is missed, patients should take it as soon as they remember. However if they miss one day of EPANOVA, they should not double the dose when they resume taking it.
Manufactured by:
Catalent Germany GmbH
Eberbach and Schorndorf, Germany
Manufactured for:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
EPANOVA is a registered trademark licensed to the AstraZeneca group of companies.
©AstraZeneca 2014
Patient Information
EPANOVA (EPP-ah-nō-vah)
(omega-3-carboxylic acids)
Capsules
What is EPANOVA?
EPANOVA is a prescription medicine used along with a low fat and low cholesterol diet to lower very high triglyceride (fat) levels in adults.
- •
- It is not known if EPANOVA changes your risk of having inflammation of your pancreas (pancreatitis).
- •
- It is not known if EPANOVA prevents you from having a heart attack or stroke.
- •
- It is not known if EPANOVA is safe and effective in children.
Who should not take EPANOVA?
Do not take EPANOVA if you are allergic to omega-3-carboxylic acids or any of the ingredients in EPANOVA. See the end of this leaflet for a complete list of ingredients in EPANOVA.
Before taking EPANOVA, tell your doctor about all your medical conditions, including if you:
- •
- have diabetes
- •
- have a low thyroid problem (hypothyroidism)
- •
- have a liver problem
- •
- have a pancreas problem
- •
- are allergic to fish or shellfish. It is not known if people who are allergic to fish or shellfish are also allergic to EPANOVA.
- •
- are pregnant or plan to become pregnant. It is not known if EPANOVA will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. EPANOVA can pass into your breast milk. You and your doctor should decide if you will take EPANOVA or breastfeed.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take EPANOVA?
- •
- Take EPANOVA exactly as your doctor tells you to take it.
- •
- Do not change your dose or stop taking EPANOVA without talking to your doctor.
- •
- If you miss a dose of EPANOVA, take it as soon as you remember. However, if you miss 1 day of EPANOVA, do not double your dose when you take it.
- •
- Take EPANOVA capsules whole. Do not break open, crush, dissolve, or chew EPANOVA capsules before swallowing. If you cannot swallow EPANOVA capsules whole, tell your doctor. You may need a different medicine.
- •
- Your doctor should start you on a diet that is low in saturated fat, cholesterol, carbohydrates, and low in added sugars before giving you EPANOVA. Stay on this diet while taking EPANOVA.
- •
- Your doctor should do blood tests to check your triglycerides, bad cholesterol and liver function levels while you take EPANOVA.
What are the possible side effects of EPANOVA?
EPANOVA may cause serious side effects, including:
- •
- increases in the results of blood tests used to check your liver function (ALT and AST) and your bad cholesterol levels (LDL-C)
- •
- possible allergic reactions if you are also allergic to fish or shellfish
The most common side effects of EPANOVA include:
- •
- diarrhea
- •
- upset stomach
- •
- abdominal pain or discomfort
- •
- burping
These are not all the possible side effects of EPANOVA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store EPANOVA?
- •
- Store EPANOVA at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Do not freeze EPANOVA.
- •
- Keep EPANOVA and all medicines out of the reach of children.
General information about the safe and effective use of EPANOVA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or doctor for information about EPANOVA that is written for health professionals. Do not use EPANOVA for a condition for which it was not prescribed. Do not give EPANOVA to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in EPANOVA?
Active Ingredient: omega-3-carboxylic acids
Inactive Ingredients: α-tocopherol (in a carrier of vegetable oil), porcine Type A gelatin, glycerol, sorbitol, and purified water (components of the capsule shell). Coating and ink components to the EPANOVA capsules also contain ethyl acrylate and methyl methacrylate copolymer dispersion, talc, titanium dioxide, iron oxide red, polysorbate 80, and carboxymethylcellulose sodium (coating components), pharmaceutical glaze, titanium dioxide, n-butyl alcohol, propylene glycol and isopropanol (ink components)
For more information, go to www.epanova.com or call 1-800-236-9933.
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued 05/2014
Manufactured by: Catalent Germany GmbH, Eberbach and Schorndorf, Germany
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE
EPANOVA is a registered trademark licensed to the AstraZeneca group of companies.
© AstraZeneca 2014
PRINCIPAL DISPLAY PANEL-BOTTLE LABEL
NDC 0310-2222-60 60 Capsules
Epanova®
(omega-3-carboxylic acids)
Capsules
Rx only 1 gram
Swallow capsule whole
AstraZeneca
Each capsule contains 1 gram omega-3-carboxylic acids with
at least 850 mg polyunsaturated free fatty acids.
Usual Adult Dosage: See accompanying Prescribing Information.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F)
[see USP Controlled Room Temperature]. Do not freeze.
EPANOVA is a registered trademark licensed to the AstraZeneca group
of companies. © AstraZeneca 2014
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
By: Catalent Germany GmbH, Eberbach and Schorndorf, Germany
Product of Canada
00000-00
PRINCIPAL DISPLAY PANEL-PROFESSIONAL SAMPLE
NDC 0310-2222-95 6 Capsules
Epanova®
(omega-3-carboxylic acids)
Capsules
1 gram per capsule
Swallow capsule whole
Rx Only
Professional Sample—Not for Sale
AstraZeneca
Each capsule contains 1 gram omega-3-carboxylic
acids with at least 850 mg polyunsaturated free
fatty acids.
Usual Adult Dosage: See accompanying
Prescribing Information.
Store at 25°C (77°F); excursions permitted to
15°-30°C (59°-86°F) [see USP Controlled Room
Temperature]. Do not freeze. Keep out of reach
of children.
EPANOVA is a registered trademark licensed to
the AstraZeneca group of companies.
© AstraZeneca 2014
Manufactured for: AstraZeneca Pharmaceuticals LP,
Wilmington, DE 19850
By: Catalent Germany GmbH, Eberbach and
Schorndorf, Germany
Product of Canada
00000-00 AstraZeneca
| EPANOVA
omega-3-carboxylic acids capsule, gelatin coated |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |