WINRHO
-
human immunoglobulin g liquid
Cangene bioPharma Inc
----------
Rho(D) Immune Globulin Intravenous (Human)WinRho® SDF
WARNING: INTRAVASCULAR HEMOLYSIS (IVH)
Intravascular hemolysis (IVH) leading to death has been reported in patients treated for immune thrombocytopenic purpura (ITP) with WinRho® SDF.
IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS).
Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least eight hours after administration. Perform a dipstick urinalysis at baseline, 2 hours, 4 hours after administration and prior to the end of the monitoring period. Alert patients and monitor the signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hematuria. Absence of these signs and/or symptoms of IVH within eight hours do not indicate IVH cannot occur subsequently. If signs and/or symptoms of IVH are present or suspected after WinRho® administration post-treatment laboratory tests should be performed including plasma hemoglobin, haptoglobin, LDH, and plasma bilirubin (direct and indirect).
DESCRIPTION
Rho(D) Immune Globulin Intravenous (Human) (Rho(D) IGIV) - WinRho® SDF - is available as a sterile, liquid gamma globulin (IgG) fraction containing antibodies to the Rho(D) antigen (D antigen). WinRho® SDF is prepared from human plasma by an anion-exchange column chromatography method.1 The manufacturing process includes a solvent detergent treatment step (using tri-n-butyl phosphate and Triton® X-100) that is effective in inactivating lipid enveloped viruses such as hepatitis B, hepatitis C, and HIV.2 WinRho® SDF is filtered using a Planova™ 20N virus filter which has been validated to be effective in the removal of some non‑lipid enveloped viruses.3 These two processes are designed to increase product safety by reducing the risk of transmission of enveloped and non-enveloped viruses, respectively.
The product potency is expressed in international units by comparison to the World Health Organization (WHO) standard. A 1,500 Unit (International Unit [IU])* (300 microgram [µg]) vial contains sufficient anti-Rho(D) to effectively suppress the immunizing potential of approximately 17 mL of Rho(D) (D-positive) red blood cells (RBCs).
The liquid formulation is stabilized with 10% maltose and 0.03% polysorbate 80. There are no preservatives in the formulation. WinRho® SDF does not contain mercury. This product contains approximately 5 micrograms/mL IgA.
* In the past, a full dose of Rho(D) Immune Globulin (Human) has traditionally been referred to as a “300 microgram” dose. Potency and dosing recommendations are now expressed in IU by comparison to the WHO anti-Rho(D) standard. The conversion of “microgram” to “Units [IU]” is: 1 microgram = 5 Units.
CLINICAL PHARMACOLOGY
Pharmacology
Treatment of Immune Thrombocytopenic Purpura (ITP)
WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), has been shown to increase platelet counts in non-splenectomized, Rho(D) positive patients with ITP. Platelet counts usually rise within one to two days and peak within seven to 14 days after initiation of therapy. The duration of response is variable; however, the average duration is approximately 30 days. The mechanism of action is not completely understood, but is thought to be due to the formation of anti-Rho(D) (anti-D)-coated RBC complexes resulting in Fc receptor blockade, thus sparing antibody-coated platelets.4,5
Suppression of Rh Isoimmunization
WinRho® SDF is used to suppress the immune response of non-sensitized Rho(D) negative individuals following exposure to Rho(D) positive RBCs by fetomaternal hemorrhage during delivery of an Rho(D) positive infant, abortion (spontaneous or induced), amniocentesis, abdominal trauma, or mismatched transfusion.6 The mechanism of action is not completely understood.
WinRho® SDF when administered within 72 hours of a full-term delivery of an Rho(D) positive infant by an Rho(D) negative mother will reduce the incidence of Rh isoimmunization from 12‑13% to 1-2%. The 1-2% is, for the most part, due to isoimmunization during the last trimester of pregnancy. When treatment is given both antenatally, at 28 weeks gestation, and postpartum, the Rh immunization rate drops to about 0.1%.7, 8
When 600 IU (120 µg) of Rho(D) IGIV is administered to pregnant women, passive anti-Rho(D) antibodies are not detectable in the circulation for more than six weeks and therefore a dose of 1,500 IU (300 µg) should be used for antenatal administration.
In a clinical study with Rho(D) negative volunteers (nine males and one female), Rho(D) positive red cells were completely cleared from the circulation within eight hours of intravenous administration of Rho(D) IGIV. There was no indication of Rh isoimmunization of these subjects at six months after the clearance of the Rho(D) positive red cells.
Pharmacokinetics
IM versus IV Administration (Lyophilized Powder)
In a clinical study involving Rho(D) negative volunteers, two subjects received 600 IU (120 µg) Rho(D) IGIV by intravenous (IV) administration and two subjects received this dose by intramuscular (IM) administration. Peak levels (36 to 48 ng/mL) were reached within two hours of IV administration and peak levels (18 to 19 ng/mL) were reached at five to 10 days after IM administration. Although no statistical comparisons were made, the calculated areas under the curve were comparable for both routes of administration. The t½ for anti-Rho(D) was about 24 days following IV administration and about 30 days following IM administration.
Lyophilized Powder versus Liquid Formulation
In two comparative pharmacokinetics studies9, 101 volunteers were administered the liquid or lyophilized formulation of WinRho® SDF intravenously (n=41) or intramuscularly (n=60). The formulations were bioequivalent following IV administration based on area under the curve to 84 days and had comparable pharmacokinetics following IM administration. The average peak concentrations (Cmax) of anti-Rho(D) for both formulations were comparable following IV or IM administration and occurred within 30 minutes or 2-4 days of administration, respectively. Both formulations also had similar elimination half-lives (t½) following IV or IM administration.
Clinical Studies
Treatment of ITP
Efficacy was documented in four subgroups of patients with ITP:
Childhood Chronic ITP
In an open-label, single arm, multicenter study, 24 non-splenectomized, Rho(D) positive children with ITP of greater than six months duration were treated initially with 250 IU/kg (50 µg/kg) Rho(D) Immune Globulin Intravenous (Human) [125 IU/kg (25 µg/kg) on days 1 and 2, with subsequent doses ranging from 125 to 275 IU/kg (25 to 55 µg/kg)]. Response was defined as a platelet increase to at least 50,000/mm3 and a doubling of the baseline. Nineteen of 24 patients responded for an overall response rate of 79%, an overall mean peak platelet count of 229,400/mm3 (range 43,300 to 456,000), and a mean duration of response of 36.5 days (range 6 to 84).10
Childhood Acute ITP
A multicenter, randomized, controlled trial comparing Rho(D) IGIV to high dose and low dose Immune Globulin Intravenous (Human) (IGIV) and prednisone was conducted in 146 non-splenectomized, Rho(D) positive children with acute ITP and platelet counts less than 20,000/mm3. Of 38 patients receiving Rho(D) IGIV [125 IU/kg (25 µg/kg) on days 1 and 2], 32 patients (84%) responded (platelet count ≥ 50,000/mm3) with a mean peak platelet count of 319,500/mm3 (range 61,000 to 892,000), with no statistically significant differences compared to other treatment arms. The mean times to achieving ≥ 20,000/mm3 or ≥ 50,000/mm3 platelets for patients receiving Rho(D) IGIV were 1.9 and 2.8 days respectively. When comparing the different therapies for time to platelet count ≥ 20,000/mm3 or ≥ 50,000/mm3, no statistically significant differences among treatment groups were detected, with a range of 1.3 to 1.9 days and 2.0 to 3.2 days, for IGIV and prednisone respectively.11, 12
Adult Chronic ITP
Twenty-four non‑splenectomized Rho(D) positive adults with ITP of greater than six months duration and platelet counts < 30,000/mm3 or requiring therapy were enrolled in a single-arm, open-label trial and treated with 100 to 375 IU/kg (20 to 75 µg/kg) Rho(D) IGIV (mean dose 231 IU/kg (46.2 µg/kg). Twenty-one of 24 patients responded (increase ≥ 20,000/mm3) during the first two courses of therapy for an overall response rate of 88% with a mean peak platelet count of 92,300/mm3 (range 8,000 to 229,000).13-15
ITP Secondary to HIV Infection
Eleven children and 52 adults, who were non-splenectomized and Rho(D) positive, with all Walter Reed classes of HIV infection and ITP, with initial platelet counts of ≤ 30,000/mm3 or requiring therapy, were treated with 100 to 375 IU/kg (20 to 75 µg/kg) Rho(D) IGIV in an open label trial. Rho(D) IGIV was administered for an average of 7.3 courses (range 1 to 57) over a mean period of 407 days (range 6 to 1,952). Fifty-seven of 63 patients responded (increase ≥ 20,000/mm3) during the first six courses of therapy for an overall response rate of 90%. The overall mean change in platelet count for six courses was 60,900/mm3 (range -2,000 to 565,000), and the mean peak platelet count was 81,700/mm3 (range 16,000 to 593,000).13-16
Suppression of Rh Isoimmunization
A pivotal study supporting this indication was conducted in 1,186 non-sensitized, Rho(D) negative pregnant women in cases in which the blood types of the fathers were Rho(D) positive or unknown. Rho(D) IGIV was administered according to one of three regimens: 1) 93 women received 600 IU (120 µg) at 28 weeks; 2) 131 women received 1200 IU (240 µg) each at 28 and 34 weeks; 3) 962 women received 1200 IU (240 µg) at 28 weeks. All women received a postnatal administration of 600 IU (120 µg) if the newborn was found to be Rho(D) positive. Of 1,186 women who received antenatal Rho(D) IGIV, 806 were given Rho(D) IGIV postnatally following the delivery of an Rho(D) positive infant, of which 325 women underwent testing at six months after delivery for evidence of Rh isoimmunization. Of these 325 women, 23 would have been expected to display signs of Rh isoimmunization, however, none was observed (p <0.001 in a Chi-square test of significance of difference between observed and expected isoimmunization in the absence of Rho(D) IGIV).
INDICATIONS AND USAGE
Treatment of ITP
WinRho® SDF must be administered via the intravenous route when used in clinical situations requiring an increase in platelet count to prevent excessive hemorrhage in the treatment of non‑splenectomized, Rho(D) positive:
- children with chronic or acute ITP,
- adults with chronic ITP, or
- children and adults with ITP secondary to HIV infection
The safety and efficacy of WinRho® have not been evaluated in clinical trials for patients with non-ITP causes of thrombocytopenia or in previously splenectomized patients or in patients who are Rho(D) negative.
Suppression of Rh Isoimmunization
Pregnancy and Other Obstetric Conditions
WinRho® SDF may be administered by either intramuscular injection or intravenously. WinRho® SDF is indicated for the suppression of Rh isoimmunization in non-sensitized, Rho(D) negative (D-negative) women within 72 hours after spontaneous or induced abortions, amniocentesis, chorionic villus sampling, ruptured tubal pregnancy, abdominal trauma or transplacental hemorrhage or in the normal course of pregnancy unless the blood type of the fetus or father is known to be Rho(D) negative. In the case of maternal bleeding due to threatened abortion, WinRho® SDF should be administered as soon as possible. Suppression of Rh isoimmunization reduces the likelihood of hemolytic disease in an Rho(D) positive fetus in present and future pregnancies. WinRho® SDF should not be administered to infants born to Rh incompatible mothers.
The criteria for an Rh-incompatible pregnancy requiring administration of WinRho® SDF at 28 weeks gestation and within 72 hours after delivery in a Rho(D) negative mother are:
- the mother is carrying a child whose father is either Rho(D) positive or Rho(D) unknown,
- the baby is either Rho(D) positive or Rho(D) unknown, and
- the mother must not be previously sensitized to the Rho(D) factor.
Transfusion
WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), is recommended for the suppression of Rh isoimmunization in Rho(D) negative female children and female adults in their childbearing years transfused with Rho(D) positive RBCs or blood components containing Rho(D) positive RBCs. Treatment should be initiated within 72 hours of exposure. Treatment should be given (without preceding exchange transfusion) only if the transfused Rho(D) positive blood represents less than 20% of the total circulating red cells. A 1,500 IU (300 µg) dose will suppress the immunizing potential of approximately 17 mL of Rho(D) positive RBCs.
WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), is not indicated for use as immunoglobulin replacement therapy for immune globulin deficiency syndromes. It should not be used for the treatment of ITP in Rho(D) negative or splenectomized individuals; efficacy in these patients has not been demonstrated.
CONTRAINDICATIONS
- Do not use WinRho® SDF in patients with known anaphylactic or severe hypersensitivity responses to human immune globulin products
- Do not use WinRho® SDF in patients with autoimmune hemolytic anemia
- Do not use WinRho® SDF in patients with pre-existing hemolysis or in patients at high risk for hemolysis
- Do not use WinRho® SDF in patients who are IgA deficient with antibodies against IgA
- Do not use WinRho® SDF in infants for the suppression of isoimmunization, Rho(D)
WARNINGS
INTRAVASCULAR HEMOLYSIS (IVH)
Intravascular hemolysis (IVH) leading to death has been reported in patients treated for immune thrombocytopenic purpura (ITP) with WinRho® SDF.
IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS).
Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least eight hours after administration. Perform a dipstick urinalysis at baseline, 2 hours, 4 hours after administration and prior to the end of the monitoring period. Alert patients and monitor for signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hematuria. Absence of these signs and/or symptoms of IVH within eight hours do not indicate IVH cannot occur subsequently. If signs and/or symptoms of IVH are present or if IVH is suspected after WinRho® administration, post-treatment laboratory tests should be performed including plasma hemoglobin, haptoglobin, LDH, and plasma bilirubin (direct and indirect).
False High Blood Glucose Levels
The liquid formulation of WinRho® SDF contains maltose. Maltose in IGIV products has been shown to give falsely high blood glucose levels in certain types of blood glucose testing systems (for example, by systems based on glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase methods). Due to the potential for falsely elevated glucose readings, only testing systems that are glucose-specific should be used to test or monitor blood glucose levels in patients receiving maltose‑containing parenteral products, including WinRho® SDF Liquid.
The product information of the blood glucose testing system, including that of the test strips, should be carefully reviewed to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, contact the manufacturer of the testing system to determine if the system is appropriate for use with maltose-containing parenteral products.
Transmissible Infectious Agents
WinRho® SDF is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses [see DESCRIPTION]. Despite these measures such products can still potentially transmit disease. Because this product is made from human blood it may carry a risk of transmitting infectious agents, e.g., viruses and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Cangene Corporation at 1-800-768-2304. The physician should discuss the risks and benefits of this product with the patient.
PRECAUTIONS
General
Hypersensitivity
Severe hypersensitivity reactions may occur [see CONTRAINDICATIONS]. In case of hypersensitivity discontinue WinRho® SDF infusion immediately and institute appropriate treatment. Medications such as epinephirine should be available for immediate treatment of acute hypersensitivity reactions.
WinRho® SDF contains approximately 5 micrograms/mL IgA. Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions. WinRho® SDF is contraindicated in patients with antibodies against IgA and a history of hypersensitivity reaction [see CONTRAINDICATIONS].
Treatment of ITP
Renal Failure
Use of Immune Globulin Intravenous (IGIV) products, particularly those containing sucrose, have been reported to be associated with renal dysfunction, acute renal failure, osmotic nephropathy, and death.17 Patients at risk of acute renal failure include those with any degree of pre-existing renal insufficiency, diabetes mellitus, advanced age (above 65 years of age), volume depletion, sepsis, paraproteinemia, or receiving known nephrotoxic drugs. WinRho® SDF does not contain sucrose.
For patients at risk of renal dysfunction or failure, administer WinRho® SDF at the minimum infusion rate practicable.
Thrombotic Events
Thrombotic events may occur following treatment with WinRho® SDF and other IGIV products.18, 19 Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, and/or known/suspected hyperviscosity.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies. For patients judged to be at risk of developing thrombotic events, administer WinRho® SDF at the minimum rate of infusion practicable.
Hemolysis
Although the mechanism of action of WinRho® SDF in the treatment of ITP is not completely understood it is postulated that anti-D binds to the Rho(D) RBC resulting in formation of antibody-coated RBC complexes. Immune-mediated clearance of the antibody-coated RBC complexes would spare the antibody-coated platelets because of the preferential destruction of antibody-coated RBC complexes by the macrophages located in the reticuloendothelial system. 4, 5, 20 The side effect of this action is a decrease in hemoglobin levels (extravascular hemolysis). The pooled data from ITP clinical studies demonstrated a maximum decrease from baseline in hemoglobin levels of 1.2 g/dL within 7 days after administration of WinRho® SDF.
If the patient has lower than normal hemoglobin levels (less than 10 g/dL), a reduced dose of 125 to 200 IU/kg (25 to 40 μg/kg) should be given to minimize the risk of increasing the severity of anemia in the patient. Alternative treatments should be used in patients with hemoglobin levels that are less than 8 g/dL due to the risk of increasing the severity of the anemia. [see DOSAGE AND ADMINISTRATION, Treatment of ITP].
Significant anemia may present with pallor, hypotension, or tachycardia while acute renal insufficiency may present with oliguria or anuria, edema and dyspnea. Patients with IVH who develop DIC may exhibit signs and symptoms of increased bruising and prolongation of bleeding time and clotting time which may be difficult to detect in the ITP population. Consequently the diagnosis of this serious complication of IVH is dependent on laboratory testing [see PRECAUTIONS: Laboratory tests]. Previous uneventful administration of WinRho® SDF does not preclude the possibility of an occurrence of IVH and its complications following any subsequent administration of WinRho® SDF. ITP patients presenting with signs and/or symptoms of IVH and its complications after anti-D administration should have confirmatory laboratory testing that may include, but is not limited to, CBC (i.e. hemoglobin, platelet counts), haptoglobin, plasma hemoglobin, urine dipstick, assessment of renal function (i.e. BUN, serum creatinine), liver function (i.e. LDH, direct and indirect bilirubin) and DIC specific tests such as D‑dimer or Fibrin Degradation Products (FDP) or Fibrin Split Products (FSP).
Patients should be instructed to immediately report symptoms of back pain, shaking, fever, discolored urine, decreased urine output, sudden weight gain, fluid retention/edema and/or shortness of breath to their physicians.
If ITP patients are to be transfused, Rho(D) negative red blood cells (PRBCs) should be used so as not to exacerbate ongoing hemolysis. Platelet products may contain up to 5.0 mL of RBCs, thus caution should likewise be exercised if platelets from Rho(D) positive donors are transfused.
Transfusion-related Acute Lung Injury
Noncardiogenic pulmonary edema may occur in patients following IGIV treatment.21
TRALI is characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever. Symptoms typically appear within 1 to 6 hours following treatment.
Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of anti-neutrophil antibodies in both the product and the patient’s serum [see PRECAUTIONS: Laboratory Tests].
TRALI may be managed using oxygen therapy with adequate ventilatory support.
Information for Patients
ITP
Instruct patients being treated with WinRho® SDF for ITP to immediately report symptoms of intravascular hemolysis including back pain, shaking chills, fever, discolored urine, decreased urine output, sudden weight gain, fluid retention/edema, and/or shortness of breath to their physicians.
Prior to discharge instruct patients to continue to self-monitor for the signs and symptoms of IVH over 72 hours, especially for discoloration of urine, and to seek medical attention immediately in the event that signs/symptoms of IVH occur following WinRho® SDF administration.
ITP and Suppression of Rh Isoimmunization
Inform patients of the early signs of hypersensitivity reactions to WinRho® SDF including hives, generalized urticaria, chest tightness, wheezing, hypotension, and anaphylaxis and advise them to notify their physician if they experience these symptoms.
Laboratory Tests
ITP
For all ITP patients, blood type, blood count, reticulocyte count, DAT and dipstick urinalysis are recommended before deciding to treat patients with WinRho® SDF. In patients with evidence of hemolysis, or patients at risk of hemolysis, other treatments should be used [see WARNINGS].
Patients administered WinRho® SDF are closely monitored for at least 8 hours post administration and a dipstick urinalysis is performed at baseline, 2 hours, 4 hours after administration and prior to the end of the monitoring period.
If signs and/or symptoms of IVH and its complications are present after anti-D administration, appropriate confirmatory laboratory testing should be done that may include, but is not limited to, CBC (i.e. hemoglobin, platelet counts), haptoglobin, plasma hemoglobin, urine dipstick, assessment of renal function (i.e. BUN, serum creatinine), liver function (i.e. LDH, direct and indirect bilirubin) and DIC specific tests such as D-dimer or Fibrin Degradation Products (FDP) or Fibrin Split Products (FSP).
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at an increased risk of developing acute renal failure [see PRECAUTIONS]. Assess renal function in these at-risk patients, including measurement of BUN and serum creatinine, before the initial infusion of WinRho® SDF and at appropriate intervals thereafter.
If TRALI is suspected, appropriate tests should be performed for the presence of anti-neutrophil antibodies in both the product and patient serum [see PRECAUTIONS].
Suppression of Rh Isoimmunization
WinRho® SDF should not be administered to Rho(D) negative individuals who are Rh immunized as evidenced by an indirect antiglobulin (Coombs’) test revealing the presence of anti-Rho(D) (anti-D) antibody.
A large fetomaternal hemorrhage late in pregnancy or following delivery may cause a weak mixed field positive Du test result. Such an individual should be assessed for a large fetomaternal hemorrhage and the dose of WinRho® SDF adjusted accordingly. The presence of passively administered anti-Rho(D) in maternal or fetal blood can lead to a positive direct antiglobulin (Coombs’) test. If there is an uncertainty about the father’s Rh group or immune status, WinRho® SDF should be administered to the mother.
Drug Interactions
Treatment of ITP and Suppression of Rh Isoimmunization
Administration of WinRho® SDF concomitantly with other drugs has not been evaluated. Other antibodies contained in WinRho® SDF may interfere with the response to live virus vaccines such as measles, mumps, polio or rubella. Therefore, immunization with live vaccines should not be given within 3 months after WinRho® SDF administration.
Drug/Laboratory Test Interactions
WinRho® SDF contains trace amounts of anti-A, anti-B, anti-C, anti-E and other blood group antibodies (for example, anti-Duffy, anti-Kidd (anti-JKa) antibodies)22 that may be detectable in direct and indirect antiglobulin (Coombs’) tests obtained following WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), administration. Interpretation of direct and indirect antiglobulin tests must be made in the context of the patient’s underlying clinical condition and supporting laboratory data.
Pregnancy Category C
Treatment of ITP and Suppression of Rh Isoimmunization
Animal reproduction studies have not been conducted with WinRho® SDF. It is also not known whether WinRho® SDF can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. WinRho® SDF should be given to a pregnant woman only if clearly needed.
Geriatric Use
Clinical studies of WinRho® SDF did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience suggests that patients of advanced age (age over 65) with co-morbid conditions such as active infection (including HCV), hematological malignancies (including non-Hodgkin’s lymphoma, Hodgkin’s disease or Chronic Lymphocytic Leukemia), autoimmune disorders (SLE, antiphosholipid syndrome, and autoimmune hemolytic anemia) may be at an increased risk of developing acute hemolytic reactions such as IVH. Patients receiving doses in excess of 300 IU/kg of WinRho® SDF may also be at an increased risk of developing increased hemolysis. Fatal outcomes associated with IVH and its complications have occurred most frequently in patients of advanced age (age over 65) with co-morbid conditions.
In general, caution should be used in dose selection for an elderly patient with consideration given to starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The most serious adverse reactions have been observed in patients receiving WinRho® SDF for treatment of ITP. These include: intravascular hemolysis (IVH), clinically compromising anemia, acute renal insufficiency, DIC, and death. [see WARNINGS.]
The most common adverse reactions observed for all indications are: headaches, chills, fevers, asthenia, pallor, diarrhea, nausea, vomiting, arthralgia, myalgia, dizziness, hyperkinesia, abdominal or back pain, hypotension, hypertension, increased LDH, somnolence, vasodilation, pruritus, rash and sweating. All adverse reactions listed occurred in ≤ 2% of WinRho® doses administered in clinical trials.
The following sections describe the adverse drug reactions observed during clinical studies for each of the labelled indications. Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a specific drug product cannot be directly compared to rates in clinical trials of another drug, and may not reflect rates observed in practice.
Treatment of ITP
In clinical trials of subjects (n=161) with childhood acute ITP, adults and children with chronic ITP, and adults and children with ITP secondary to HIV, 60/848 (7%) of WinRho® infusions had at least one adverse reaction, with no adverse drug reactions reported in more than 2% of infusions. The most common adverse reactions were headache (19 infusions; 2%), chills (14 infusions; < 2%), and fever (nine infusions; 1%), which are expected adverse drug reactions following intravenous administration of human immune globulins.
WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), is administered to Rho(D) positive patients with ITP. Therefore, side effects related to the destruction of Rho(D) positive red blood cells, most notably a decreased hemoglobin, can be expected. In four clinical trials of patients treated with the recommended initial intravenous dose of 250 IU/kg (50 µg/kg) the mean maximum decrease in hemoglobin was 1.70 g/dL (range: +0.40 to –6.1g/dL). At a reduced dose, ranging from 125 to 200 IU/kg (25 to 40 µg/kg), the mean maximum decrease in hemoglobin was 0.81 g/dL (range: +0.65 to –1.9 g/dL). Only 5/137 (3.7%) of patients had a maximum decrease in hemoglobin of greater than 4 g/dL (range: -4.2 to -6.1 g/dL).
The mean maximum decrease in hemoglobin in patients who were not transfused with PRBCs was 3.7 g/dL (range: 0.0 to 7.6 g/dL). Transfusions for treatment-associated anemia were administered within hours to days of the onset of IVH and consisted of between 1-6 units of PRBCs. Acute renal insufficiency was noted within 2 to 48 hours of the onset of IVH. The mean maximum increase in serum creatinine was 3.5 mg/dL (range: 0.8 to 10.3 mg/dL) and occurred within 2-9 days. The renal insufficiency in all surviving patients resolved with medical management, including dialysis, within 4-23 days.
Suppression of Rh Isoimmunization
Adverse reactions to Rho(D) Immune Globulin Intravenous (Human) are rare in Rho(D) negative individuals (<0.1%)23. In the clinical trial of 1,186 Rho(D) negative pregnant women, no adverse reactions were reported to Rho(D) IGIV.
Post-marketing
ITP
The following post-marketing adverse reactions are reported voluntarily from a population of uncertain size; hence, it is not possible to estimate their frequency. These adverse reactions are classified by system organ class.
Intravascular hemolysis (IVH) leading to death has been reported in patients treated for immune thrombocytopenic purpura (ITP) with WinRho® SDF.
Serious complications including severe anemia, acute renal insufficiency, renal failure and disseminated intravascular coagulation (DIC) have also been reported.
Blood and lymphatic system disorders: Intravascular hemolysis, Disseminated Intravascular Coagulation, Hemoglobinemia
Cardiac disorders: Cardiac arrest, Cardiac failure, Myocardial infarction, Tachycardia
Gastrointestinal disorders: Nausea
General disorders and administration site conditions: Chest pain, Fatigue, Edema
Hepatobiliary disorders: Jaundice
Immune system disorders: Anaphylactic reaction, Hypersensitivity
Musculoskeletal and connective tissue disorders: Myalgia, Muscle spasm, Pain in extremities
Renal and urinary disorders: Renal failure, Renal impairment, Anuria, Chromaturia, Hemoglobinuria, Hematuria
Respiratory, thoracic, and mediastinal disorders: Acute respiratory distress syndrome, Transfusion related acute lung injury
Skin and subcutaneous tissue disorders: Hyperhidrosis
Suppression of Rh Isoimmunization
The following post-marketing adverse reactions are reported voluntarily from a population of uncertain size; hence, it is not possible to estimate their frequency. These adverse reactions are classified by system organ class.
General disorders and administration site conditions: Injection site reactions (includes induration, pruritus or swelling at injection site)
Immune system disorders: Hypersensitivity, Anaphylactic reaction
Skin and subcutaneous tissue disorders: Pruritus, Rash
Healthcare professionals should report serious adverse reactions following the administration of WinRho® SDF to Cangene Corporation at 1-800-768-2304 or FDA’s MedWatch reporting system by phone (1-800-FDA-1088).
OVERDOSAGE
Treatment of ITP and Suppression of Rh Isoimmunization
In post-marketing spontaneous reporting there has been a limited number of medication error reports related to dosage calculations in which higher doses than that recommended for WinRho® SDF were administered. These calculation errors arose due to confusion between µg and IU (1 µg = 5 IU), confusion between kilograms and pounds and miscalculation of required dosage following a large feto-maternal hemorrhage. Adverse reactions reported in ITP patients have included chills, fever, headache and larger hemoglobin decreases while no hemolytic reactions were reported in suppression of Rh isoimmunization patients. In one ITP case report that involved an overdose due to confusion between µg and IU, a patient with significant co-morbidities developed IVH and had a fatal outcome. In the event of overdose patients should be monitored closely for signs and symptoms of hemolysis and the treatment should be symptomatic and supportive.
DOSAGE AND ADMINISTRATION
Parenteral products such as WinRho® SDF should be inspected for particulate matter and discoloration prior to administration. Discard any unused portion.
Preparation and Handling
Liquid WinRho® SDF
There is no reconstitution required. Table 1 describes the target fill volumes for each of the dosage sizes for the liquid presentation of WinRho® SDF.
| Vial Size | Target Fill Volume |
| 600 IU (120 µg) | 0.5 mL |
| 1,500 IU (300 µg) | 1.3 mL |
| 2,500 IU (500 µg) | 2.2 mL |
| 5,000 IU (1,000 µg) | 4.4 mL |
| 15,000 IU (3,000 µg) | 13.0 mL |
Note: The entire contents of the vial should be removed to obtain the labeled dosage of WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human). If partial vials are required for dosage calculation, the entire contents of the vial should be withdrawn to ensure accurate calculation of the dosage requirement.
Treatment of ITP
Intravenous use only.
The entire dose of WinRho® SDF may be injected into a suitable vein as rapidly as over three to five minutes. WinRho® SDF should be administered separately from other drugs.
Initial Dosing: After confirming that the patient is Rho(D) positive, an initial dose of 250 IU/kg (50 µg/kg) body weight, given as a single injection, is recommended for the treatment of ITP. The initial dose may be administered in two divided doses given on separate days, if desired. If the patient has a hemoglobin level less than 10 g/dL, a reduced dose of 125 to 200 IU/kg (25 to 40 µg/kg) should be given to minimize the risk of increasing the severity of anemia in the patient. All patients should be monitored to determine clinical response by assessing platelet counts, red cell counts, hemoglobin, and reticulocyte levels [see PRECAUTIONS, Treatment of ITP].
Subsequent Dosing: If subsequent therapy is required to elevate platelet counts, an intravenous dose of 125 to 300 IU/kg (25 to 60 µg/kg) body weight of WinRho® SDF is recommended. The frequency of dosing and the dose used in maintenance therapy should be determined by the patient’s clinical response by assessing platelet counts, red cell counts, hemoglobin, and reticulocyte levels.
If a patient responded to initial dose with a satisfactory increase in platelets:
Maintenance Therapy:
Dosing [125 to 300 IU/kg (25 to 60 µg/kg)] individualized based on platelet and Hgb levels.
If patient did not respond to initial dose, administer a subsequent dose based on Hgb:
If Hgb between 8-10 g/dL, redose between 125 to 200 IU/kg (25 to 40 µg/kg).
If Hgb >10 g/dL, redose between 250 to 300 IU/kg (50 to 60 µg/kg).
If Hgb < 8 g/dL, alternative treatments should be used.
The following equations are provided to determine the dosage and number of vials needed for the treatment of ITP:
- weight in lbs/2.2083 = weight in kg
- weight in kg X selected IU (µg) dosing level = dosage
- dosage / vial size = number of vials needed
Safety and efficacy of WinRho® SDF in the treatment of ITP at doses exceeding 300 IU/kg (60µg/kg) has not been established.
Suppression of Rh Isoimmunization
Intravenous or intramuscular use.
For intravenous administration the entire dose of WinRho® SDF may be injected into a suitable vein as rapidly as over three to five minutes. WinRho® SDF should be administered separately from other drugs.
For intramuscular administration, administer into the deltoid muscle of the upper arm or the anterolateral aspects of the upper thigh. Due to the risk of sciatic nerve injury, the gluteal region should not be used as a routine injection site. If the gluteal region is used, use only the upper, outer quadrant.
Pregnancy and other Obstetric Indications
Table 2 provides dosing guidelines based on the condition being treated.
|
||
| Indication | Timing of Administration |
Dose (Administer IM or IV) |
| Rh-incompatible Pregnancy: | ||
| Routine antepartum prophylaxis | 28 weeks gestation* | 1,500 IU (300 µg) |
|
Postpartum (if newborn Rh positive) | Within 72 hours of birth† | 600 IU (120 µg) |
| Obstetric Conditions: | ||
| Threatened abortion at any time | Immediately | 1,500 IU (300 µg) |
| Amniocentesis and chorionic villus sampling before 34 weeks gestation | Immediately after procedure‡ | 1,500 IU (300 µg) |
| Abortion, amniocentesis, or any other manipulation after 34 weeks gestation | Within 72 hours | 600 IU (120 µg) |
Transfusion
WinRho® SDF should be administered within 72 hours after exposure for treatment of incompatible blood transfusions or massive fetal hemorrhage.
| Route of Administration | WinRho® SDF Dose | |
|
If exposed to Rho(D) Positive Whole Blood: |
If exposed to Rho(D) Positive Red Blood Cells: |
|
| Intravenous | 45 IU (9 µg)/mL blood | 90 IU (18 µg)/mL cells |
| Intramuscular | 60 IU (12 µg)/mL blood | 120 IU (24 µg)/mL cells |
Administer 3,000 IU (600 µg) every 8 hours via the intravenous route until the total dose, calculated from the above table, is administered.
Administer 6,000 IU (1,200 µg) every 12 hours via the intramuscular route until the total dose, calculated from the above table, is administered.
HOW SUPPLIED
WinRho® SDF, Rho(D) Immune Globulin Intravenous (Human), is available in packages containing:
Liquid
| NDC Number |
Contents |
| 53270-3120-1 |
A box containing a single dose vial of 600 IU (120 µg) anti-Rho(D) |
| 53270-3300-1 |
A box containing a single dose vial of 1,500 IU (300 µg) anti-Rho(D) |
| 53270-3500-1 |
A box containing a single dose vial of 2,500 IU (500 µg) anti-Rho(D) |
| 53270-3100-1 |
A box containing a single dose vial of 5,000 IU (1,000 µg) anti-Rho(D) |
| 53270-3000-1 |
A box containing a single dose vial of 15,000 IU (3,000 µg) anti-Rho(D) |
STORAGE
Store at 2 to 8°C (36 to 46°F). Do not freeze. Do not use after expiration date.
If the reconstituted product is not used immediately, store it at room temperature for no longer than 12 hours. Do not freeze the reconstituted product. Discard the product if not administered within 12 hours.
REFERENCES
- Bowman, JM, et al.: WinRho: Rh immune globulin prepared by ion exchange for intravenous use. Can. Med. Assoc. J. 1980; 123:1121-1125.
- Horowitz, B: Investigations into the application of tri(n-butyl)phosphate/detergent mixtures to blood derivatives. Morgenthaler J (ed): Virus Inactivation in Plasma Products, Curr. Stud. Hematol. Blood. Transfus. 1989; 56:83-96.
- Soluk L, et al.: Pathogen safety of intravenous Rh immunoglobulin liquid and other immune globulin products: enhanced nanofiltration and manufacturing process overview. Am J Ther 2008; 15:435-43.
- Ballow, M: Mechanisms of action of intravenous immunoglobulin therapy and potential use in autoimmune connective tissue diseases. Cancer. 1991; 68:1430-1436.
- Kniker, WT: Immunosuppressive agents, γ-globulin, immunomodulation, immunization, and apheresis. J. Aller. Clin. Immunol. 1989; 84:1104-1106.
- Bowman, JM: Suppression of Rh isoimmunization: a review. Obstet. & Gynec. 1978; 52:385-393.
- Bowman, JM, and Pollock, JM: Failures of intravenous Rh immune globulin prophylaxis: An analysis of the reasons for such failures. Trans. Med. Rev. 1987; 1:101-111.
- Bowman, JM: Antenatal suppression of Rh alloimmunization. Clin Obstet. & Gynec. 1991; 34:296-303.
- Sinclair CJ, et al.: Comparative pharmacokinetics of liquid and lyophilized formulations of IV RhIG immune globulin. Biologicals 2008; 36:256-62.
- Andrew, M, et al.: A multicenter study of the treatment of childhood chronic idiopathic thrombocytopenic purpura with anti-D. J Pediatrics 1992; 120:522-527.
- Blanchette, V, et al.: Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet 1994; 344: 703-707.
- Zunich KM, et al. Intravenous anti-D immunoglobulin for childhood acute immune thrombocytopenic purpura. Lancet 1995; 346:1363-5.
- Scaradavou A, et al.: Intravenous anti-D treatment of immune thrombocytopenic purpura: experience in 272 patients. Blood 1997; 89:2689-700.
- Bussel, JB, et al.: Intravenous anti-D treatment of immune thrombocytopenic purpura: Analysis of efficacy, toxicity, and mechanism of effect. Blood 1991; 77: 1884-1893.
- Bussel, JB, et al.: IV anti-D treatment of ITP: Results in 210 cases. Abstract, The American Society of Hematology, Anaheim, CA, December 1992.
- Zunich KM, et al.: Treatment of human immunodeficiency virus-related thrombocytopenia with intravenous anti-rhesus D immunoglobulin. Clin Infect Dis 1996; 22:1129-30.
- Gupta N, Ahmed I, Nissel-Horowitz S, Patel D, Mehrotra B. Intravenous gammaglobulin-associated acute renal failure. Am J Hematol 2001; 66:151-152
- Dalakas MC. High-dose intravenous immunoglobulin and serum viscosity: risk of precipitating thromboembolic events. Neurology 1994; 44:223-226.
- Woodruff RK, et al.: Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet 1986; 2:217-218.
- Lazarus AH, Crow AR. Mechanism of action of IVIG and anti-D in ITP. Transfus Apheresis Sci 2003; 28:249-255.
- Rizk A, et al.: Transfusion-related acute lung injury after the infusion of IVIG. Transfusion 2001; 41:264-8.
- Rushin J, Rumsey, DH, Ewing, CA, Sandler, SG. Detection of multiple passively acquired alloantibodies following infusions of IV Rh immune globulins. Transfusion Vol. 40, May 2000.
- CIOMS. Current challenges in Pharmacovigilance: Pragmatic Approaches. Report of CIOMS Working Group V. Geneva 2001. Page 122.
Manufactured by:
Cangene Corporation
Winnipeg, Manitoba
Canada R3T 5Y3
U.S. License No. 1201
Distributed by:
Cangene bioPharma, Inc.
Baltimore, MD
21230 USA
To report adverse events contact Cangene Corporation at 1-800-768-2304
Triton® is trademark of Rohm & Haas Company
Planova™ is a trademark of Asahi Kasei Kogyo Kabushiki Kaisha Corporation.
Date of Revision: March 2010
Part No. 35024300
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF
INFORMATION FOR PATIENTS BEING TREATED FOR ITP
You should read this leaflet carefully each time before you are scheduled to receive a treatment for your Immune Thrombocytopenic Purpura (ITP) with WinRho® SDF. This leaflet is a summary of the important information you need to know about your medicine, and does not take the place of talking with your doctor and does not contain all of the information available about WinRho® SDF. If you have any questions after reading this leaflet, make sure you ask your doctor or nurse.
1. WHAT IS WinRho® SDF? Pronounced, “Win Row S D F”
WinRho® SDF is a medicine that belongs to the group of medicines called immune therapy and is used to treat people with the bleeding disorder called ITP. ITP is a bleeding disorder caused by an abnormally low number of platelets. Platelets are found in the bloodstream and are needed for your blood to clot properly. When blood does not clot properly, there is a tendency to bruise and bleed easily.
WinRho® SDF is also used as a form of protection against the development of antibodies in a person with Rh-negative blood who is given Rh-positive blood, and in pregnancy to prevent an Rh-negative mother’s immune system from destroying an Rh-positive baby’s red blood cells.
2. HOW DOES WinRho® SDF WORK?
WinRho® SDF is a medicine that contains antibodies. Antibodies are made by your body’s immune system and help your body fight infections caused by bacteria and viruses and defend your body against other foreign substances. When your immune system is working properly, the antibodies made by your body coat the bacteria, viruses or foreign substances, which are then removed by an organ in your abdomen called the spleen. But, sometimes, these antibodies can also attack the healthy cells in your body, which is what happens when you have ITP. In ITP, the body mistakenly produces antibodies against its own platelets. These antibodies coat your platelets, and the spleen removes them so the number of platelets in your blood stream decreases.
WinRho® SDF is thought to protect the platelets of Rh-positive people by coating their red blood cells, causing the red blood cells to be removed by the spleen instead of the platelets. As a result, there is an increased number of platelets in your blood and fewer symptoms of ITP. But, because your red blood cells are being removed, you could become severely anemic (see WHAT IS THE MOST IMPORTANT INFORMATION I NEED TO KNOW ABOUT TREATMENT WITH WinRho® SDF for ITP?)
3. WHAT IS THE MOST IMPORTANT INFORMATION I NEED TO KNOW ABOUT TREATMENT WITH WinRho® SDF for ITP?
A small decrease in the amount of red blood cells is expected after treatment with WinRho® SDF. However, a small number of patients have experienced a potentially life threatening reaction in which a large number of red blood cells are destroyed while in the blood stream. In the patients that experienced this reaction, most had symptoms within 4 hours of receiving WinRho® SDF.
If you experience any of the following symptoms after receiving WinRho® SDF, you should seek medical attention immediately:
- shaking chills, fever or back pain,
- discolored or darkened urine,
- decreased urine production,
- swelling,
- shortness of breath.
If you have a condition that causes on-going red blood cell destruction (hemolytic anemia) you should not use WinRho® SDF to treat your ITP.
If you have been told that you have an IgA deficiency, you have a greater risk of having an allergic reaction to WinRho® SDF. While there is only a rare chance that you may experience a sudden, severe allergic reaction after receiving WinRho® SDF, you should be aware of the early symptoms of an allergic reaction. These are:
- hives,
- rash,
- chest tightness,
- wheezing,
- shortness of breath,
- feeling light-headed or dizzy when you stand (this could mean a drop in blood pressure).
If you experience any of these symptoms, seek medical attention immediately.
WinRho® SDF is prepared from donated human plasma. When products of this type are administered, the possibility of passing on infection from the donors can not be totally ruled out. This also applies to viruses or infections that are not yet known. A number of measures are taken to reduce the risk of passing on infection/viruses by WinRho® SDF including careful selection of blood and plasma donors to make sure those at risk of carrying infections/viruses can not donate, and the testing of each donation and the pools of plasma for signs of viruses such as AIDS virus HIV, hepatitis B virus and hepatitis C virus. The manufacturing process for WinRho® SDF also includes a number of steps that remove or inactivate viruses such as a solvent/detergent step and a special filter for removing viruses.
4. WHAT ARE THE MOST COMMON SIDE EFFECTS OF WinRho® SDF?
Like all medicines, WinRho® SDF can have side effects.
The most common side effects of WinRho® SDF are muscle pain or tenderness at the injection site, chills, skin reactions (rash and itching), fever and headache.
5. WHO SHOULD NOT USE WinRho® SDF
If you have had a severe allergic reaction such as swelling of the airway, difficulty breathing, or feeling light-headed or dizzy when you stand (drop in blood pressure), after receiving WinRho® SDF or other human immune globulins, or have been told you have an IgA deficiency, you should tell your doctor before you are given WinRho® SDF. Your doctor may choose another treatment for you.
If you know your blood type and you are Rh-negative, or if you are Rh-positive and have had your spleen surgically removed, you should not be given WinRho® SDF.
6. CAN I GET WinRho® SDF IF I AM TAKING OTHER MEDICINES?
Tell your doctor or healthcare provider that will be giving you the injection of WinRho® SDF if you are taking or have recently taken other prescription or over the counter medicines, and any supplements.
You should tell your doctor if you have recently been vaccinated or are planning to be vaccinated. WinRho® SDF may interfere with the response to certain vaccines (e.g. measles, rubella, mumps, and chicken pox) and it may be necessary to delay vaccination.
WinRho® SDF can interfere with certain blood tests. If you have a blood test after your WinRho® SDF injection, tell the person taking your blood or your doctor that you have received WinRho® SDF.
7. HOW CAN I ACCESS CANGENE’S PATIENT RESOURCES?
You can contact Cangene to receive more product information.
Product information Hotline: 1-800-4WINRHO (1-800-494-6746)
Product Website: www.winrho.com
You can call Cangene at 1-800-768-2304 to receive more information on patient assistance programs available to you.
Principal Display Panel - WinRho SDF Liquid 600 IU
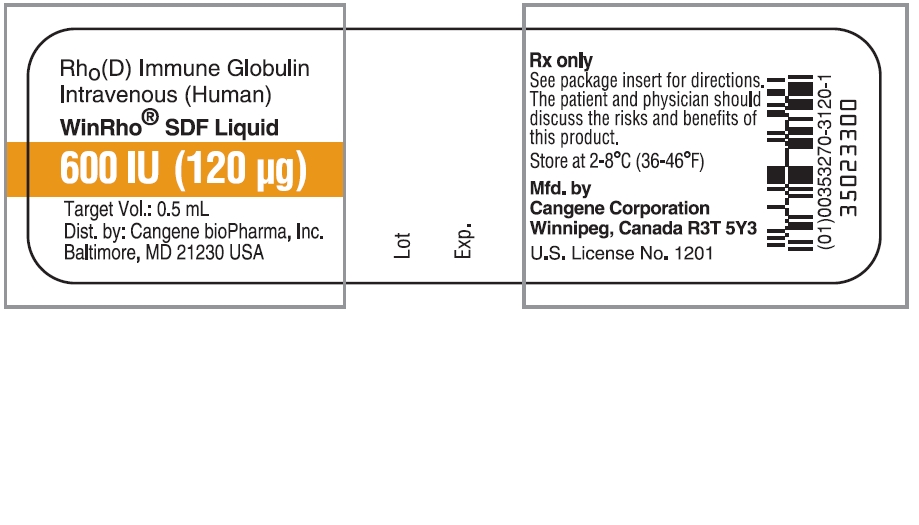
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
600 IU (120 µg)
Target Vol.: 0.5 mL
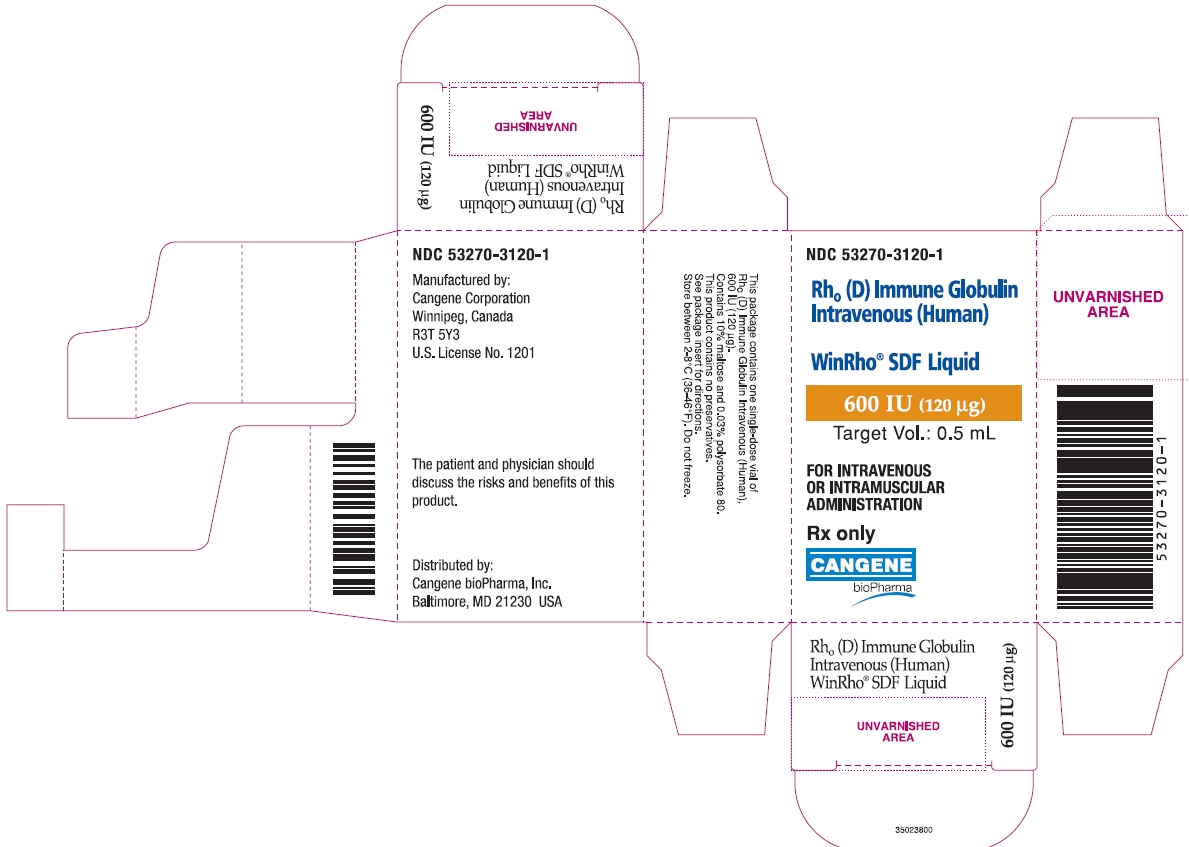
NDC 53270-3120-1
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
600 IU (120 µg)
Target Vol.: 0.5 mL
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
Principal Display Panel - WinRho SDF Liquid 1500 IU
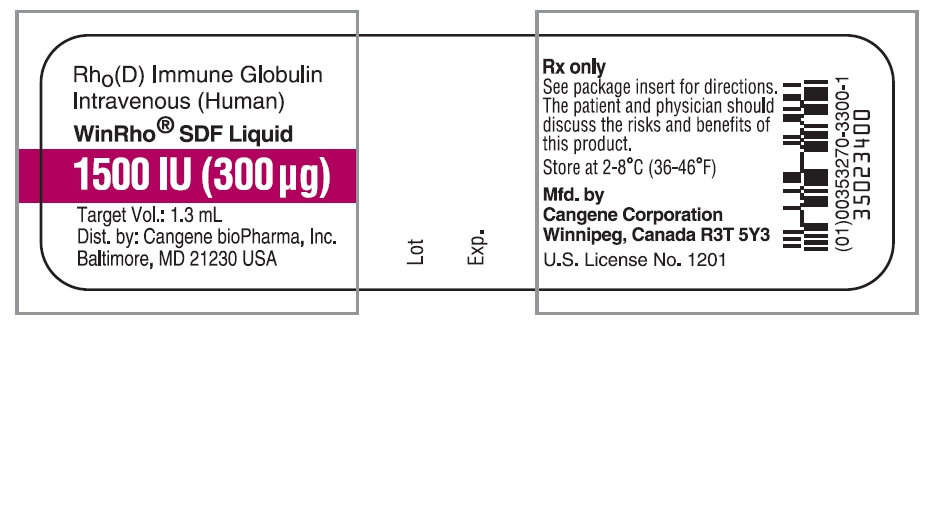
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
1500 IU (300 µg)
Target Vol.: 1.3 mL
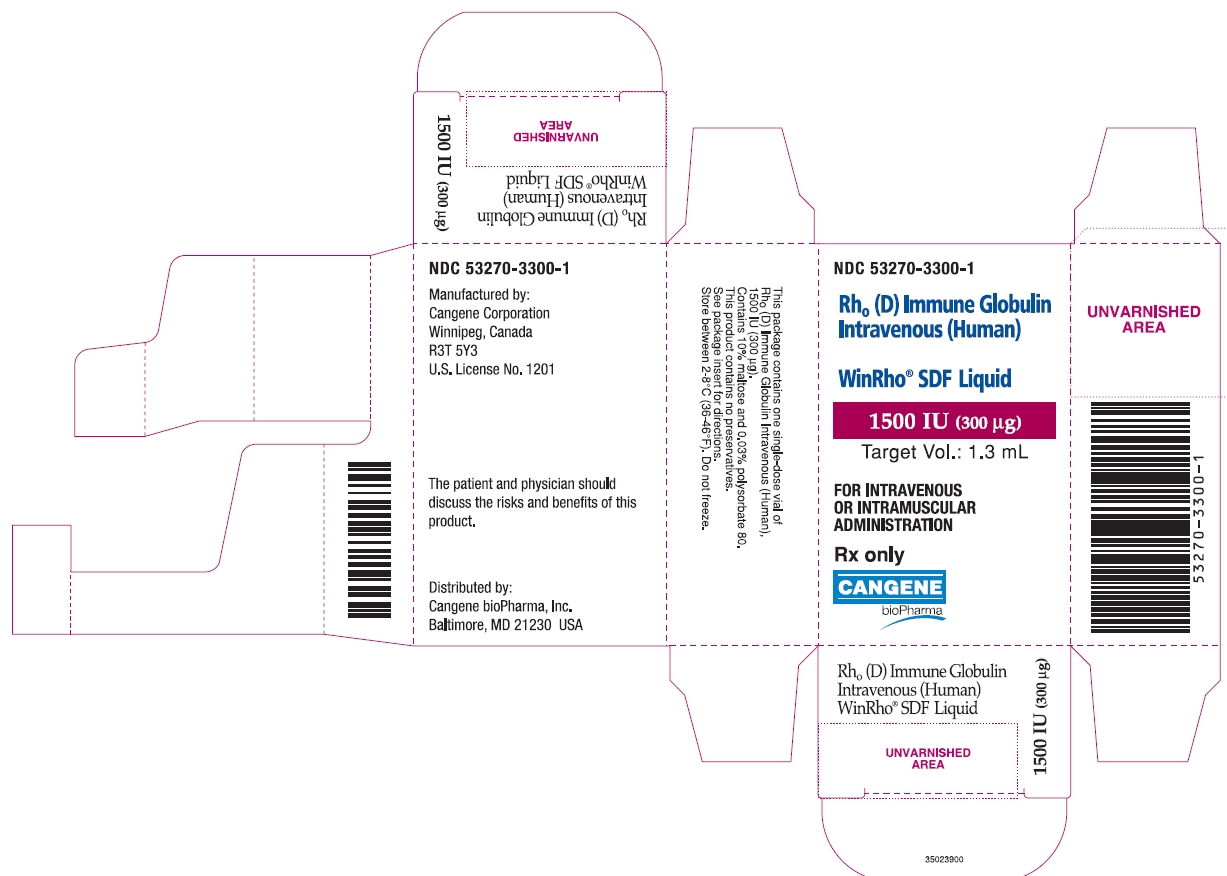
NDC 53270-3300-1
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
1500 IU (300 µg)
Target Vol.: 1.3 mL
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
Principal Display Panel - WinRho SDF Liquid 2500 IU
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
2500 IU (500 µg)
Target Vol.: 2.2 mL
NDC 53270-3500-1
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
2500 IU (500 µg)
Target Vol.: 2.2 mL
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
Principal Display Panel - WinRho SDF Liquid 5000 IU
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
5000 IU (1000 µg)
Target Vol.: 4.4 mL
NDC 53270-3100-1
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
5000 IU (1000 µg)
Target Vol.: 4.4 mL
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
Principal Display Panel - WinRho SDF Liquid 15000 IU
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
15000 IU (3000 µg)
Target Vol.: 13.0 mL
NDC 53270-3000-1
Rho(D) Immune Globulin Intravenous (Human)
WinRho® SDF Liquid
15000 IU (3000 µg)
Target Vol.: 13 mL
FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION
Rx only
| WINRHO
rho (d) immune globulin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103649 | 03/31/2005 | |
| WINRHO
rho (d) immune globulin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103649 | 03/31/2005 | |
| WINRHO
rho (d) immune globulin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103649 | 03/31/2005 | |
| WINRHO
rho (d) immune globulin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103649 | 03/31/2005 | |
| WINRHO
rho (d) immune globulin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103649 | 03/31/2005 | |
| Labeler - Cangene bioPharma Inc (050783398) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cangene Corporation | 244844056 | ANALYSIS, MANUFACTURE | |