CLINDA-DERM- clindamycin phosphate solution
Paddock Laboratories, LLC
----------
Clinda-Derm™
Clindamycin Phosphate
Topical Solution USP, 1%
Description
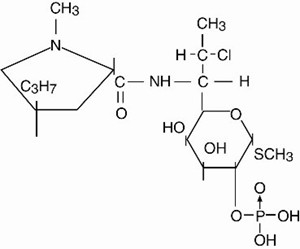
Clindamycin Phosphate C18H34ClN2O8PS
(MW = 504.96)
Clindamycin phosphate topical solution, for external use, contains clindamycin phosphate at a concentration equivalent to 10 mg clindamycin per milliliter in a hydroalcoholic solution. Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
The chemical name for clindamycin phosphate is 7(S)-chloro-7-deoxylincomycin-2-phosphate.
Each mL contains clindamycin phosphate equivalent to 10 mg clindamycin. In addition, each mL contains isopropyl alcohol (51.5% v/v), propylene glycol, sodium hydroxide and purified water.
CLINICAL PHARMACOLOGY:
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Clindamycin has been shown to have in vitro activity against isolates of Propionibacterium acnes. This may account for its usefulness in acne.
Cross resistance has been demonstrated between clindamycin and lincomycin.
Antagonism has been demonstrated between clindamycin and erythromycin.
Following multiple topical applications of clindamycin phosphate at a concentration equivalent to 10 mg clindamycin per mL in an isopropyl alcohol and water solution, very low levels of clindamycin are present in the serum (0-3 ng/mL) and less than 0.2% of the dose is recovered in urine as clindamycin.
Clindamycin activity has been demonstrated in comedones from acne patients. Clindamycin in vitro inhibits all Propionibacterium acnes cultures tested (MICs 0.4 mcg/mL). Free fatty acids on the skin surface have been decreased from approximately 14% to 2% following application of clindamycin.
INDICATIONS AND USAGE:
Clinda-Derm is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether other agents are more appropriate. (See CONTRAINDICATIONS, WARNINGS, and ADVERSE REACTIONS.)
CONTRAINDICATIONS:
Clinda-Derm is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis.
WARNINGS:
Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally. Use of the topical formulation results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin. Symptoms can occur after a few days, weeks or months following initiation of clindamycin therapy. They have also been observed to begin up to several weeks after cessation of therapy with clindamycin. Studies indicate a toxin(s) produced by Clostridium difficile is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis.
When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered in cases of severe diarrhea.
Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen the condition. Vancomycin has been found to be effective in the treatment of antibiotic-associated pseudomembranous colitis produced by Clostridium difficile. The usual adult dosage is 500 mg to 2 grams of vancomycin orally per day in three to four divided doses administered for 7 to 10 days.
Mild cases of colitis may respond to discontinuance of clindamycin. Moderate to severe cases should be managed promptly with fluid, electrolyte, and protein supplementation as indicated. Cholestyramine and colestipol resins have been shown to bind the toxin in vitro. If both a resin and vancomycin are to be administered concurrently, it may be advisable to separate the time of administration of each drug. Systemic corticoids and corticoid retention enemas may help relieve the colitis. Other causes of colitis should also be considered. A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
PRECAUTIONS:
General:
Clinda-Derm (clindamycin phosphate topical solution) contains an alcoholic base which will cause burning and irritation of the eye. In the event of accidental contact with sensitive surfaces (eye, abraded skin, mucous membranes), bathe with copious amounts of cool tap water. The solution has an unpleasant taste and caution should be exercised when applying medication around the mouth.
Clindamycin phosphate topical solution should be prescribed with caution in atopic individuals.
Pregnancy Category B:
Reproduction studies have been performed in rats and mice using subcutaneous and oral doses of clindamycin ranging from 100 mg to 600 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to clindamycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers:
It is not known whether clindamycin is excreted in human milk following use of Clinda-Derm (clindamycin phosphate topical solution). However, orally and parentally administered clindamycin has been reported to appear in breast milk. As a general rule, nursing should not be undertaken while a patient is on a drug since many drugs are excreted in human milk.
ADVERSE REACTIONS:
Skin dryness is the most common adverse reaction seen with the solution.
Clindamycin has been associated with severe colitis which may end fatally (see WARNINGS).
Cases of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with topical formulations of clindamycin.
Other effects which have been reported in association with the use of topical formulations of clindamycin include:
|
Abdominal pain |
Irritation |
|
Contact dermatitis |
Oily Skin |
|
Gastrointestinal disturbances |
Sensitization |
|
Gram-negative folliculitis |
Stinging of the eye |
| CLINDA-DERM
clindamycin phosphate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Paddock Laboratories, LLC (967694121) |