GLUCAGEN HYPOKIT- glucagon hydrochloride
Novo Nordisk
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GlucaGen safely and effectively. See full prescribing information for GlucaGen.
GlucaGen® (glucagon [rDNA origin] for injection) Initial U.S. Approval: 1998 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONTreatment of severe hypoglycemia (GlucaGen HypoKit)
Use as a diagnostic aid (GlucaGen Diagnostic Kit and GlucaGen 10-Pack)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSAdverse reactions seen with GlucaGen are:
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-800-727-6500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Treatment of severe hypoglycemia
GlucaGen is used to treat severe hypoglycemic (low blood sugar) reactions which may occur in patients with diabetes mellitus treated with insulin. Because GlucaGen depletes glycogen stores, the patient should be given supplemental carbohydrates as soon as he/she awakens and is able to swallow, especially children or adolescents. Medical evaluation is recommended for all patients who experience severe hypoglycemia.
1.2 Use as a diagnostic aid
GlucaGen is indicated for use during radiologic examinations to temporarily inhibit movement of the gastrointestinal tract. Glucagon is as effective for this examination as are the anticholinergic drugs. However, the addition of the anticholinergic agent may result in increased side effects. After the end of the diagnostic procedure, give oral carbohydrates to patients who have been fasting, if this is compatible with the diagnostic procedure applied.
2 DOSAGE AND ADMINISTRATION
For GlucaGen HypoKit:
2.1 Treatment of severe hypoglycemia
- 1.
- Using the supplied prefilled syringe, carefully insert the needle through the rubber stopper of the vial containing GlucaGen powder and inject all the liquid from the syringe into the vial.
- 2.
- Shake the vial gently until the powder is completely dissolved and no particles remain in the fluid. The reconstituted fluid should be clear and of water-like consistency.
- 3.
- The reconstituted GlucaGen gives a concentration of approximately 1 mg/mL glucagon.
- 4.
- The reconstituted GlucaGen should be used immediately after reconstitution.
- 5.
- Inject 1 mL (adults and children, weighing more than 55 lbs (25 kg)) or 0.5 mL (children weighing less than 55 lbs (25 kg)) subcutaneously, intramuscularly, or intravenously. If the weight is not known: children younger than 6 years should be given a 0.5 mL and children 6 years and older should be given 1 mL.
- 6.
- Discard any unused portion.
- 7.
- Emergency assistance should be sought immediately after subcutaneous or intramuscular injection of glucagon.
- 8.
- The glucagon injection may be repeated using a new kit while waiting for emergency assistance.
- 9.
- Intravenous glucose MUST be administered if the patient fails to respond to glucagon.
- 10.
- When the patient has responded to the treatment, give oral carbohydrates to restore the liver glycogen and prevent recurrence of hypoglycemia.
- For GlucaGen Diagnostic Kit and the GlucaGen 10-pack:
2.2 Use as a diagnostic aid
1. GlucaGen should be reconstituted with 1 mL of Sterile Water for Reconstitution (if supplied) or 1 mL of Sterile Water for Injection, USP. Using a syringe, withdraw all of the Sterile Water for Reconstitution (if supplied) or 1 mL Sterile Water for Injection, USP and inject into the GlucaGen vial.
2. Shake the vial gently until the powder is completely dissolved and no particles remain in the fluid. The reconstituted fluid should be clear and of water-like consistency.
3. The reconstituted GlucaGen gives a concentration of approximately 1 mg/mL glucagon.
4. The reconstituted GlucaGen should be used immediately after reconstitution.
5. GlucaGen must be administered by medical personnel.
6. Discard any unused portion.
7. Onset of action after an injection will depend on the organ under examination and route of administration [see Pharmacodynamics (12.2)].
8. The usual diagnostic dose for relaxation of the stomach, duodenal bulb, duodenum, and small bowel is 0.2 mg to 0.5 mg given intravenously or 1 mg given intramuscularly; the usual dose to relax the colon is 0.5 mg to 0.75 mg intravenously and 1 mg to 2 mg intramuscularly [see Pharmacodynamics (12.2)].
9. After the end of the diagnostic procedure, give oral carbohydrates to patients who have been fasting, if this is compatible with the diagnostic procedure applied.
The GlucaGen Diagnostic Kit and the GlucaGen 10-pack presentations are intended only for use by healthcare providers as a diagnostic aid. The GlucaGen Diagnostic Kit and the GlucaGen 10-pack presentations are not intended for use by patients to treat severe hypoglycemia because they are not packaged with a syringe and diluent necessary for rapid preparation and administration during an emergency outside of a healthcare facility.
3 DOSAGE FORMS AND STRENGTHS
GlucaGen is supplied in a vial, alone, or accompanied by Sterile Water for Reconstitution (1 mL) also in a vial (10 pack or diagnostic kit). It is also supplied as GlucaGen HypoKit®, a presentation with a disposable prefilled syringe containing 1 mL Sterile Water for Reconstitution. When the glucagon powder is reconstituted with Sterile Water for Reconstitution (if supplied) or with Sterile Water for Injection, USP, it forms a solution of 1 mg/mL (1 unit/mL) glucagon for subcutaneous, intramuscular, or intravenous injection.
4 CONTRAINDICATIONS
GlucaGen is contraindicated in patients with:
- •
- Known hypersensitivity to glucagon, lactose or any other constituent in GlucaGen
- •
- Pheochromocytoma [see Warnings and Precautions (5.1)]
- •
- Insulinoma [see Warnings and Precautions (5.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Pheochromocytoma
Glucagon is contraindicated in patients with pheochromocytoma because Glucagon may stimulate the release of catecholamines from the tumor. If the patient develops a dramatic increase in blood pressure, 5 to 10 mg of phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed.
5.2 Insulinoma and Glucagonoma
GlucaGen should be administered cautiously to patients suspected of having insulinoma or glucagonoma. In patients with insulinoma, intravenous administration of glucagon may produce an initial increase in blood glucose; however, glucagon administration may directly or indirectly (through an initial rise in blood glucose) stimulate exaggerated insulin release from an insulinoma. A patient developing symptoms of hypoglycemia after a dose of glucagon should be given glucose orally or intravenously, whichever is most appropriate. Caution should also be observed in administering GlucaGen to patients with glucagonoma.
5.3 Hypersensitivity and Allergic Reactions
Allergic reactions may occur and include generalized rash, and in some cases anaphylactic shock with breathing difficulties, and hypotension. The anaphylactic reactions have generally occurred in association with endoscopic examination during which patients often received other agents including contrast media and local anesthetics. The patients should be given standard treatment for anaphylaxis including an injection of epinephrine if they encounter respiratory difficulties after GlucaGen injection.
5.4 Glycogen Stores and Hypoglycemia
In order for GlucaGen treatment to reverse hypoglycemia, adequate amounts of glucose must be stored in the liver (as glycogen). Therefore, GlucaGen should be used with caution in patients with conditions such as prolonged fasting, starvation, adrenal insufficiency or chronic hypoglycemia because these conditions result in low levels of releasable glucose in the liver and an inadequate reversal of hypoglycemia by GlucaGen treatment.
6 ADVERSE REACTIONS
Side effects may include nausea and vomiting at doses above 1 mg or with rapid injection. Hypotension has been reported up to 2 hours after administration in patients receiving GlucaGen as premedication for upper GI endoscopy procedures. Glucagon exerts positive inotropic and chronotropic effects and may, therefore, cause tachycardia and hypertension. Adverse reactions indicating toxicity of GlucaGen have not been reported. A temporary increase in both blood pressure and pulse rate may occur following the administration of glucagon. Patients taking beta-blockers might be expected to have a greater increase in both pulse and blood pressure, an increase of which will be temporary because of glucagon’s short half-life [see Drug Interactions (7.1)]. The increase in blood pressure and pulse rate may require therapy in patients with pheochromocytoma or coronary artery disease [see Warnings and Precautions (5.1)]. Anaphylactic reactions may occur in some cases [see Warnings and Precautions (5.3)].
The following adverse reactions have been identified during postapproval use of GlucaGen. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency.
|
Treatment of severe hypoglycemia |
|
|
Frequency (%) |
Adverse Reaction |
|
< 10 |
Nausea |
|
< 1 |
Vomiting |
|
Use as a diagnostic aid |
|
|
< 10 |
Nausea |
|
< 1 |
Vomiting |
|
< 1 |
Hypoglycemia |
|
<1 |
Hypoglycemic coma |
7 DRUG INTERACTIONS
7.1 Beta-blockers
Patients taking beta-blockers might be expected to have a greater increase in both pulse and blood pressure, an increase of which will be temporary because of glucagon’s short half-life. The increase in blood pressure and pulse rate may require therapy in patients with pheochromocytoma or coronary artery disease.
7.2 Indomethacin
When used with indomethacin, glucagon may lose its ability to raise blood glucose or may even produce hypoglycemia. Therefore, caution should be exercised for patients taking indomethacin when glucagon will be administered.
7.3 Anticholinergic Drugs
Coadministration with an anticholinergic drug is not recommended due to increased gastrointestinal side effects.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies were performed in rats and rabbits at GlucaGen doses of 0.4, 2.0, and 10 mg/kg. These doses represent exposures of up to 100 and 200 times the human dose based on mg/m2 for rats and rabbits, respectively, and revealed no evidence of harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Glucagon does not cross the human placenta barrier.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when GlucaGen is administered to a nursing woman. No clinical studies have been performed in nursing mothers, however, GlucaGen is a peptide and intact glucagon is not absorbed from the GI tract. Therefore, even if the infant ingested glucagon it would be unlikely to have any effect on the infant. Additionally, GlucaGen has a short plasma half-life thus limiting amounts available to the child. Glucagon does not cross the human placental barrier.
10 OVERDOSAGE
No reports of overdosage with GlucaGen have been reported. If overdosage occurs, the patient may experience nausea, vomiting, inhibition of GI tract motility, increase in blood pressure and pulse rate. In case of suspected overdosing, the serum potassium may decrease and should be monitored and corrected if needed. If the patient develops a dramatic increase in blood pressure, phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed.
11 DESCRIPTION
GlucaGen (glucagon [rDNA origin] for injection) is an antihypoglycemic agent and a gastrointestinal motility inhibitor. It is produced by expression of recombinant DNA in a Saccharomyces cerevisiae vector with subsequent purification. The chemical structure of the glucagon in GlucaGen is identical to human glucagon and to glucagon extracted from beef and pork pancreas. Glucagon with the empirical formula of C153H225N43O49S, and a molecular weight of 3483, is a single-chain polypeptide containing 29 amino acid residues. The structure of glucagon is:
GlucaGen is a sterile, lyophilized white powder in a 2 mL vial. The reconstituted solution contains glucagon as hydrochloride 1 mg/mL (1 unit/mL) and lactose monohydrate (107 mg). GlucaGen is supplied at pH 2.5-3.5 and is soluble in water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Antihypoglycemic Action: Glucagon induces liver glycogen breakdown, releasing glucose from the liver. Hepatic stores of glycogen are necessary for glucagon to produce an antihypoglycemic effect.
Gastrointestinal Motility Inhibition: Extra hepatic effects of glucagon include relaxation of the smooth muscle of the stomach, duodenum, small bowel, and colon.
12.2 Pharmacodynamics
For the treatment of severe hypoglycemia:
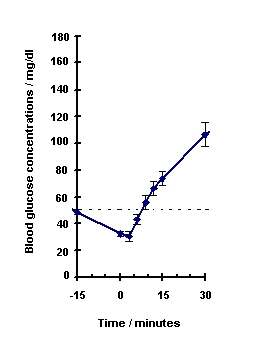
Blood glucose concentration rises within 10 minutes of injection and maximal concentrations are attained at approximately 30 minutes after injection (see Figure 1). The duration of hyperglycemic action after intravenous or intramuscular injection is 60 – 90 minutes.
Figure 1. Recovery from Insulin Induced Hypoglycemia (mean blood glucose) After Intramuscular Injection of 1 mg GlucaGen in Type I Diabetic Men
For use as a diagnostic aid:
|
||||
|
Route of Administration |
Dose* |
Time of Maximal Glucose Concentration |
Time of Onset of Action for GI Smooth Muscle Relaxation |
Duration of Smooth Muscle Relaxation† |
|
IV |
0.25-0.5 mg (0.25-0.5 units) |
5-20 minutes |
45 seconds |
9-17 minutes |
|
2 mg (2 units) |
5-20 minutes |
45 seconds |
22-25 minutes |
|
|
IM |
1 mg (1 unit) |
30 minutes |
8-10 minutes |
12-27 minutes |
|
2 mg (2 units) |
30 minutes |
4-7 minutes |
21-32 minutes |
|
12.3 Pharmacokinetics
Intramuscular injection of 1 mg GlucaGen resulted in a mean Cmax (CV%) of 1686 pg/mL (43%) and median Tmax of 12.5 minutes. The mean apparent half-life of 45 minutes after intramuscular injection probably reflects prolonged absorption from the injection site. Glucagon is degraded in the liver, kidney, and plasma.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals to evaluate carcinogenic potential have not been performed. Several studies have been conducted to evaluate the mutagenic potential of glucagon. The mutagenic potential tested in the Ames and human lymphocyte assays, was borderline positive under certain conditions for both glucagon (pancreatic) and glucagon (rDNA) origin. In vivo, very high doses (100 and 200 mg/kg) of glucagon (both origins) gave a slightly higher incidence of micronucleus formation in male mice but there was no effect in females. The weight of evidence indicates that GlucaGen is not different from glucagon pancreatic origin and does not pose a genotoxic risk to humans. GlucaGen (rDNA origin) was not tested in animal fertility studies. Studies in rats have shown that pancreatic glucagon does not cause impaired fertility.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
GlucaGen is supplied as a sterile, lyophilized white powder in a vial, alone, or accompanied by Sterile Water for Reconstitution also in a vial (Diagnostic Kit or 10-pack). It is also supplied as GlucaGen HypoKit with a disposable prefilled syringe containing Sterile Water for Reconstitution.
GlucaGen HypoKit includes:
1 vial containing 1 mg (1 unit) GlucaGen (glucagon [rDNA origin] for injection)
1 disposable syringe containing 1 mL Sterile Water for Reconstitution
NDC 0169-7065-15
GlucaGen Diagnostic Kit (NDC 0597-0260-10) includes:
1 vial containing 1 mg (1 unit) GlucaGen(glucagon [rDNA origin] for injection)
NDC 0597-0053-01
1 vial containing 1 mL Sterile Water for Reconstitution
NDC 0597-0265-94
GlucaGen Diagnostic Kit is also available as (NDC 55390-004-01) and includes:
1 vial containing 1 mg (1 unit) GlucaGen(glucagon [rDNA origin] for injection)
NDC 55390-004-01
1 vial containing 1 mL Sterile Water for Reconstitution
GlucaGen HypoKit (Two-Pack) includes: 2 packs of GlucaGen HypoKit,
Each HypoKit contains:
1 vial containing 1 mg (1 unit) GlucaGen (glucagon [rDNA origin] for injection)
1 disposable syringe containing 1 mL Sterile Water for Reconstitution
NDC 0169-7065-21
The GlucaGen 10-pack includes:
10 vials, each containing 1 mg (1 unit) GlucaGen (glucagon [rDNA origin] for injection)
NDC 55390-004-10
NDC 0597-0053-45
16.2 Recommended Storage
Before Reconstitution:
The GlucaGen package may be stored up to 24 months at controlled room temperature 20o to 25o C (68o to 77o F) prior to reconstitution. Do not freeze. Keep in the original package to protect from light. GlucaGen should not be used after the expiry date on the vials.
After Reconstitution:
Reconstituted GlucaGen should be used immediately. Discard any unused portion. If the solution shows any sign of gel formation or particles, it should be discarded.
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling (Patient Instructions for Emergency Use).]
17.1 Physician Instructions
Refer patients and family members to the FDA-approved patient labeling for instructions describing the method of preparing and injecting GlucaGen. Advise the patient and family members to become familiar with the technique of preparing glucagon before an emergency arises. Instruct patients to use 1 mg for adults or ½ the adult dose (0.5 mg) for children weighing less than 55 lb (25 kg). To prevent severe hypoglycemia, patients and family members should be informed of the symptoms of mild hypoglycemia and how to treat it appropriately. Family members should be informed to arouse the patient as quickly as possible because prolonged hypoglycemia may result in damage to the central nervous system. Patients should be advised to inform their physician when hypoglycemic reactions occur so that the treatment regimen may be adjusted if necessary.
No studies on the effects on the ability to drive and use machines have been performed. After diagnostic procedures, hypoglycemia has been reported infrequently. The patient’s ability to concentrate and react may be impaired as a result of hypoglycemia. This may present a risk in situations where these abilities are especially important, such as driving or operating machinery. Therefore, these activities should be avoided until the patient has had a meal with oral carbohydrates.
Date of issue: December 12, 2014
Version: 6
GlucaGen® and HypoKit® are registered trademarks of Novo Nordisk A/S
© 1998-2014 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-800-727-6500
www.novonordisk-us.com
Manufactured by:
Novo Nordisk A/S
2880 Bagsvaerd, Denmark
INFORMATION FOR PATIENTS
INSTRUCTIONS FOR EMERGENCY USE
GlucaGen® (Glū-ka-Gen)
(glucagon [rDNA origin] for injection)
HypoKit®
1. GlucaGen® HypoKit® is for use in case of emergency for very low blood sugar (severe hypoglycemia) in people with diabetes who use insulin.
2. Read and become familiar with the following instructions before an emergency happens. If you have questions about using GlucaGen® HypoKit® talk with your doctor or pharmacist.
What is the most important information I should know about GlucaGen® HypoKit®?
- 1. Make sure that your family members, coworkers, or close friends know that if you become unconscious they should use this kit right away. They should call for emergency medical help immediately after using the kit.
- 2. Show your family members and others where you keep this kit and how to use it. They need to know how to use it before you need it. They can practice giving a shot by giving you your normal insulin shots. It is important that they practice. A person who has never given a shot may not be able to give one in an emergency.
- 3. It is important that your family member or friend:
- •
- Act quickly. Being unconscious for a period of time may be harmful. Follow these instructions to give GlucaGen® the right way.
- •
- Turn the unconscious person on their side to prevent choking.
- •
- Do not mix GlucaGen® until they are ready to use it.
- •
- Mix the GlucaGen®. The syringe does not have GlucaGen® in it. You must mix the contents of the syringe with GlucaGen® in the vial provided in GlucaGen® HypoKit® before giving the injection. (See Instructions for Use)
- •
- Throw away any mixed GlucaGen® that is not used.
The patient may be in a coma from very high blood sugar (severe hyperglycemia), rather than very low blood sugar. If this is the case, the unconscious person will not respond to GlucaGen®.
What is GlucaGen®?
GlucaGen® is a prescription medicine used to treat very low blood sugar that can happen in people who have diabetes and use insulin.
Symptoms of severe hypoglycemia include: confusion, loss of consciousness, and seizures.
You should only give GlucaGen® injection if:
- 1. the person is unconscious or
- 2. the person is having a seizure or
- 3. the person is confused and unable to eat sugar or a sugar-sweetened product.
GlucaGen® may have been prescribed so that your friends or family members can give the injection if you become hypoglycemic and are unable to take sugar by mouth.
Less severe cases of hypoglycemia should be treated right away by eating sugar or a sugar-sweetened product such as a regular soft drink or fruit juice.
GlucaGen® does not work if it is taken by mouth.
Who should not use GlucaGen®?
Do not use GlucaGen® if the person:
- •
- is allergic to either glucagon or lactose
- •
- has a tumor of the adrenal gland called a pheochromocytoma
- •
- has a tumor of the pancreas called insulinoma
Instructions for Use:
To Prepare GlucaGen® For Injection:
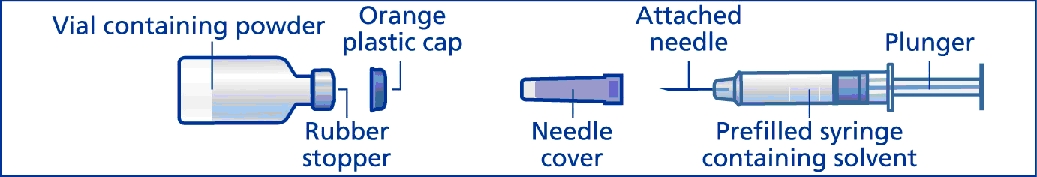
GlucaGen® HypoKit® includes:
- •
- 1 vial containing 1 mg GlucaGen® powder (glucagon [rDNA origin] for injection)
- •
- 1 prefilled disposable syringe with attached needle containing 1 mL solvent (sterile water) for reconstitution
The vial with GlucaGen® in it has a protective plastic cap. You must remove the plastic cap to inject the water and mix the GlucaGen®. If the cap is loose or missing when you buy the package, return it to your local pharmacy.
Use the prefilled disposable syringe that comes in GlucaGen® HypoKit®, with the attached needle, to mix the GlucaGen® and sterile water before giving the injection.
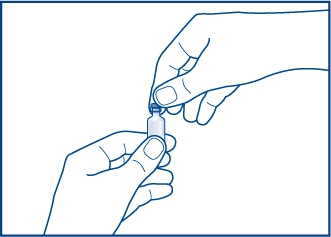
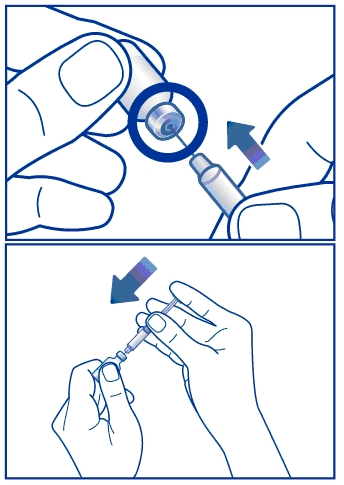
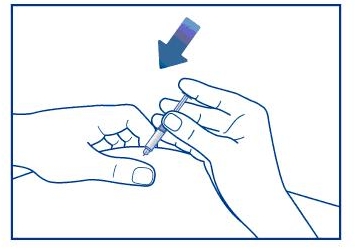
Step 1. Take the orange plastic cap off the vial of GlucaGen®. See Figure A.
Figure A
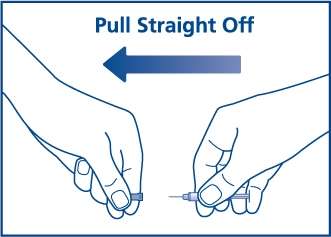
Step 2. Pull the needle cover off the needle. See Figure B.
Figure B
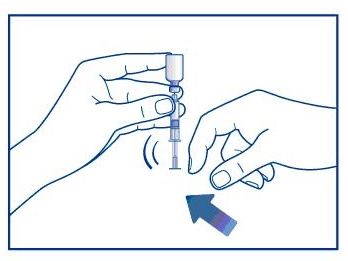
Step 3. Insert the needle through the rubber stopper (within the marked circle) of the vial containing GlucaGen® and inject all the liquid from the syringe into the vial. See Figure C.
Figure C
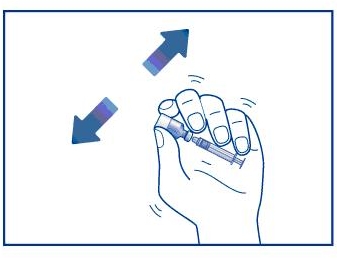
Step 4. Without taking the syringe and needle out of the vial, gently shake the vial in your hand until the powder dissolves completely. See Figure D. The liquid should be clear. Do not use if the liquid has any particles in it or it is not clear.
Figure D
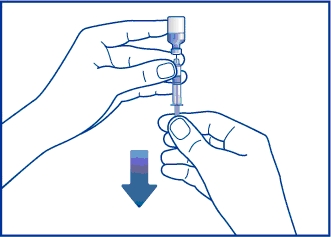
Step 5. Without taking the syringe and needle out of the vial, turn the vial upside down. Keep the needle in the liquid. Gently pull back on the plunger and slowly withdraw all the liquid into the syringe. See Figure E. Do not pull the plunger out of the syringe. This helps prevent the medicine from leaking out of the syringe.
Figure E
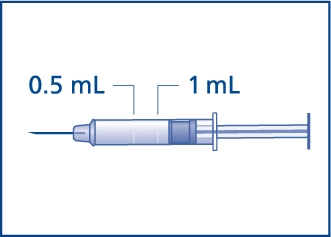
- •
- The usual dose for adults and children who weigh more than 55 pounds (25 kg) is 1 mg (1 mL). Withdraw the liquid to 1 mL mark on the syringe. See Figure F.
- •
- The usual dose for children who weigh less than 55 pounds (25 kg) is 0.5 mg (half of the adult dose). Withdraw the liquid to the 0.5 mL mark on the syringe for these children. See Figure F.
Figure F
If you do not know how much the child weighs:
- •
- Give a child under 6 years of age 0.5 mg (0.5 mL).
- •
- Give a child 6 years of age and older 1 mg (1 mL).
Step 6. Keep the needle in the liquid inside the vial. Remove any air bubbles in the syringe by flicking the syringe with your finger. See Figure G. Gently push on the plunger to move any air bubbles out of the syringe and into the vial.
Figure G
Continue pushing the plunger until you have the correct dose as described in Step 5. If the plunger is pushed below the needed dose, pull back the plunger until you have the correct dose. When you have a correct amount of GlucaGen® in the syringe, take the syringe with the attached needle out of the vial.
To Inject GlucaGen®:
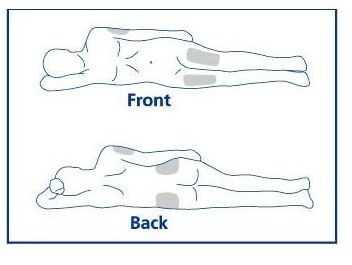
Step 7. See Figure H for commonly used injection sites. Choose an area for injection.
Common injection sites
Figure H
Insert the needle into the loose tissue at the injection site, and inject the GlucaGen®. See Figure I.
Figure I
After Giving the GlucaGen® Injection:
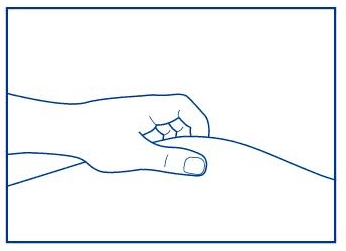
Step 8. Withdraw the needle and press on the injection site. See Figure J.
Figure J
Place the used syringe with attached needle in a sharps container (such as a red biohazard container), a hard plastic container (such as a detergent bottle), or a metal container (such as an empty coffee can). Seal the container and throw it away the right way. There may be state and local laws about the right way to throw away used syringes and needles. Ask your doctor or pharmacist how to throw away used syringes and needles. Throw away any GlucaGen® you do not use.
Step 9. Turn the person on their side. When an unconscious person awakens, they may vomit. Turning the person on their side will lessen the chance of choking.
Step 10. Call for emergency medical help right away.
Step 11. Feed the person as soon as they are awake and able to swallow. Give the person a fast-acting source of sugar (such as a regular soft drink or fruit juice) and a long-acting source of sugar (such as crackers and cheese or a meat sandwich). If the person does not awaken within 15 minutes, give another dose of GlucaGen® using a new kit.
Step 12. Even if the GlucaGen® treatment awakens the person, tell their doctor right away. The doctor should be told whenever a severe hypoglycemia reaction happens. The person’s dose of diabetes medicine may need to be changed.
Hypoglycemia may happen again after receiving GlucaGen® treatment.
Early symptoms of hypoglycemia may include:
|
|
If not treated early, hypoglycemia may worsen and the person may have severe hypoglycemia. Signs of severe hypoglycemia include:
- •
- confusion
- •
- unconsciousness
- •
- seizures
- •
- death
Pregnancy. GlucaGen® is for use only during sudden, severe hypoglycemic attacks.
Nursing Mothers. It is not known if GlucaGen® passes into your breast milk. GlucaGen® does not stay in your body very long. Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
GlucaGen® may affect the way other medicines work, and other medicines may affect how GlucaGen® works.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
What are the possible side effects of GlucaGen®?
GlucaGen® may cause serious side effects including:
- •
- allergic reactions. Symptoms of a severe allergic reaction may include:
- •
- rash or itching
- •
- raised red patches on your skin (hives)
- •
- swelling of the face, lips, tongue or throat
- •
- problems breathing or swallowing
- •
- very high or very low blood pressure
- •
- fast or slow heartbeat
The most common side effects of GlucaGen® include:
- •
- nausea
- •
- vomiting
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of GlucaGen®. For more information ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store GlucaGen® HypoKit®?
Before mixing:
- •
- Store GlucaGen® HypoKit® for up to 24 months at 68°F to 77°F (20°C to 25°C).
- •
- Do not freeze GlucaGen® HypoKit®.
- •
- Keep GlucaGen® HypoKit® in the original package to protect from light.
- •
- Do not use GlucaGen® HypoKit® after the expiration date printed on the package.
After mixing:
- •
- Mixed GlucaGen® should be used right away.
- •
- GlucaGen® and the syringe with sterile water that comes in the kit, do not contain preservatives and should only be used one time. Throw away any unused medicine.
Keep GlucaGen® HypoKit®, all medicines, needles and syringes out of the reach of children.
What are the ingredients in the GlucaGen® HypoKit®?
Active Ingredient: glucagon [rDNA origin] for injection
Inactive ingredients: lactose, sterile water for reconstitution
Date of Issue: April 25, 2014
Version: 3
GlucaGen® and HypoKit® are registered trademarks of Novo Nordisk A/S
© 1998-2014 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-800-727-6500
www.novonordisk-us.com
Manufactured by:
Novo Nordisk A/S
2880 Bagsvaerd, Denmark
PRINCIPAL DISPLAY PANEL
novo nordisk®
NDC 0169-7065-15
GlucaGen® HypoKit
(glucagon [rDNA origin] for injection)
Emergency Use for Low Blood Sugar
1 mg per vial
Single use only. Discard unused portion.
Protect from light
Rx only
This kit contains:
1 vial GlucaGen® for injection 1mg,
1 prefilled syringe of Sterile Water for
Reconstitution – for use as diluent
After reconstitution, the resulting GlucaGen®
solution contains 1mg/mL of Glucagon.
IMPORTANT - Read the enclosed insert
carefully for directions before using.
Usual Dose - 1 mg per vial for subcutaneous,
intramuscular or intravenous injection in
adults and pediatric patients (55 lbs or greater).
Smaller pediatric patients see enclosed insert.
Mix immediately before use.
Add the entire content of the accompanying
syringe of Sterile Water for Reconstitution
into the GlucaGen® vial.
Mix well and gently withdraw the entire
contents of the vial back into the syringe.
NON-RETURNABLE
Before Mixing: Store at controlled room
temperature 20° to 25° C (68° to 77° F)
Novo Nordisk Inc.
Plainsboro, NJ 08536
www.novonordisk-us.com
1-800-727-6500
Manufactured by:
Novo Nordisk A/S
2880 Bagsvaerd, Denmark
8-9402-31-201-4
Exp/Lot:
Principal Display Panel
novo nordisk®
GlucaGen® HypoKit®
NDC 0169-7065-21
(Contains 2 of NDC 0169-7065-15)
LIST 706521
(glucagon [rDNA origin] for injection)
Emergency Use for Low Blood Sugar
1 mg per vial
Single use only. Discard unused portion
Protect from light
Rx only
This carton contains 2 packs of GlucaGen® HypoKit®.
Each kit contains: 1 vial GlucaGen® for injection 1 mg,
1 prefilled syringe of Sterile Water for Reconstitution – for use as diluent
After reconstitution, the resulting GlucaGen® solution contains 1 mg/mL of Glucagon.
IMPORTANT – Read the enclosed insert carefully for directions before using.
Carton contains 2 packs of GlucaGen® HypoKit®
| GLUCAGEN HYPOKIT
glucagon hydrochloride kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Novo Nordisk (622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S | 306711800 | MANUFACTURE(0169-7065) , API MANUFACTURE(0169-7065) | |