ENBREL
-
etanercept solution
ENBREL
-
etanercept
Immunex Corp
----------
ENBREL®(etanercept)
For Subcutaneous Injection
WARNINGS
SERIOUS INFECTIONS
Patients treated with ENBREL® are at increased risk for developing serious infections that may lead to hospitalization or death (see WARNINGS and ADVERSE REACTIONS). Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
ENBREL® should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Patients should be tested for latent tuberculosis before ENBREL® use and during therapy. Treatment for latent infection should be initiated prior to ENBREL® use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens.
The risks and benefits of treatment with ENBREL® should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with ENBREL®, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCIES
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including ENBREL®.
DESCRIPTION
ENBREL® (etanercept) is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. The Fc component of etanercept contains the CH2 domain, the CH3 domain and hinge region, but not the CH1 domain of IgG1. Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kilodaltons.
ENBREL® single-use prefilled syringes are available in 25 mg (0.51 mL of a 50 mg/mL solution of etanercept) and 50 mg (0.98 mL of a 50 mg/mL solution of etanercept) dosage strengths.
ENBREL® single-use prefilled SureClick® autoinjectors are available in 50 mg (0.98 mL of a 50 mg/mL solution of etanercept).
The solution of ENBREL® is clear and colorless, sterile, preservative-free, and is formulated at pH 6.3 ± 0.2. Each ENBREL® prefilled syringe and SureClick autoinjector contains a 50 mg/mL solution of etanercept with 1% sucrose, 100 mM sodium chloride, 25mM L-arginine hydrochloride, and 25mM sodium phosphate.
ENBREL® multiple-use vials are available containing 25 mg of etanercept. ENBREL® is supplied in a multiple-use vial as a sterile, white, preservative-free, lyophilized powder. Reconstitution with 1 mL of the supplied Sterile Bacteriostatic Water for Injection (BWFI), USP (containing 0.9% benzyl alcohol) yields a multiple-use, clear, and colorless solution with a pH of 7.4 ± 0.3 containing 25 mg etanercept, 40 mg mannitol, 10 mg sucrose, and 1.2 mg tromethamine.
Administration of one 50 mg ENBREL® prefilled syringe or one ENBREL® SureClick autoinjector provides a dose equivalent to two 25 mg ENBREL® prefilled syringes or two multiple-use vials of lyophilized ENBREL®, when vials are reconstituted and administered as recommended.
CLINICAL PHARMACOLOGY
General
Etanercept binds specifically to tumor necrosis factor (TNF) and blocks its interaction with cell surface TNF receptors. TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. It plays an important role in the inflammatory processes of rheumatoid arthritis (RA), polyarticular-course juvenile idiopathic arthritis (JIA), and ankylosing spondylitis and the resulting joint pathology. In addition, TNF plays a role in the inflammatory process of plaque psoriasis. Elevated levels of TNF are found in involved tissues and fluids of patients with RA, psoriatic arthritis, ankylosing spondylitis (AS), and plaque psoriasis.
Two distinct receptors for TNF (TNFRs), a 55 kilodalton protein (p55) and a 75 kilodalton protein (p75), exist naturally as monomeric molecules on cell surfaces and in soluble forms. Biological activity of TNF is dependent upon binding to either cell surface TNFR.
Etanercept is a dimeric soluble form of the p75 TNF receptor that can bind to two TNF molecules. It inhibits the activity of TNF in vitro and has been shown to affect several animal models of inflammation, including murine collagen-induced arthritis. Etanercept inhibits binding of both TNFα and TNFβ (lymphotoxin alpha [LTα]) to cell surface TNFRs, rendering TNF biologically inactive. Cells expressing transmembrane TNF that bind ENBREL® are not lysed in vitro in the presence or absence of complement.
Etanercept can also modulate biological responses that are induced or regulated by TNF, including expression of adhesion molecules responsible for leukocyte migration (i.e., E-selectin and to a lesser extent intercellular adhesion molecule-1 [ICAM-1]), serum levels of cytokines (e.g., IL-6), and serum levels of matrix metalloproteinase-3 (MMP-3 or stromelysin).
Pharmacokinetics
After administration of 25 mg of ENBREL® by a single subcutaneous (SC) injection to 25 patients with RA, a mean ± standard deviation half-life of 102 ± 30 hours was observed with a clearance of 160 ± 80 mL/hr. A maximum serum concentration (Cmax) of 1.1 ± 0.6 mcg/mL and time to Cmax of 69 ± 34 hours was observed in these patients following a single 25 mg dose. After 6 months of twice weekly 25 mg doses in these same RA patients, the mean Cmax was 2.4 ± 1.0 mcg/mL (N = 23). Patients exhibited a two- to seven-fold increase in peak serum concentrations and approximately four-fold increase in AUC0-72 hr (range 1 to 17 fold) with repeated dosing. Serum concentrations in patients with RA have not been measured for periods of dosing that exceed 6 months. The pharmacokinetic parameters in patients with plaque psoriasis were similar to those seen in patients with RA.
In another study, serum concentration profiles at steady state were comparable among patients with RA treated with 50 mg ENBREL® once weekly and those treated with 25 mg ENBREL® twice weekly. The mean (± standard deviation) Cmax, Cmin, and partial AUC were 2.4 ± 1.5 mcg/mL, 1.2 ± 0.7 mcg/mL, and 297 ± 166 mcg•h/mL, respectively, for patients treated with 50 mg ENBREL® once weekly (N = 21); and 2.6 ± 1.2 mcg/mL, 1.4 ± 0.7 mcg/mL, and 316 ± 135 mcg•h/mL for patients treated with 25 mg ENBREL® twice weekly (N = 16).
Pharmacokinetic parameters were not different between men and women and did not vary with age in adult patients. No formal pharmacokinetic studies have been conducted to examine the effects of renal or hepatic impairment on ENBREL® disposition.
Patients with JIA (ages 4 to 17 years) were administered 0.4 mg/kg of ENBREL® twice weekly for up to 18 weeks. The mean serum concentration after repeated SC dosing was 2.1 mcg/mL, with a range of 0.7 to 4.3 mcg/mL. Limited data suggests that the clearance of ENBREL® is reduced slightly in children ages 4 to 8 years. Population pharmacokinetic analyses predict that administration of 0.8 mg/kg of ENBREL® once weekly will result in Cmax 11% higher, and Cmin 20% lower at steady state as compared to administration of 0.4 mg/kg of ENBREL® twice weekly. The predicted pharmacokinetic differences between the regimens in JIA patients are of the same magnitude as the differences observed between twice weekly and weekly regimens in adult RA patients.
CLINICAL STUDIES
Adult Rheumatoid Arthritis
The safety and efficacy of ENBREL® were assessed in four randomized, double-blind, controlled studies. The results of all four trials were expressed in percentage of patients with improvement in RA using American College of Rheumatology (ACR) response criteria.
Study I evaluated 234 patients with active RA who were ≥ 18 years old, had failed therapy with at least one but no more than four disease-modifying antirheumatic drugs (DMARDs; e.g., hydroxychloroquine, oral or injectable gold, methotrexate [MTX], azathioprine, D-penicillamine, sulfasalazine), and had ≥ 12 tender joints, ≥ 10 swollen joints, and either ESR ≥ 28 mm/hr, CRP > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg ENBREL® or placebo were administered SC twice a week for 6 consecutive months. Results from patients receiving 25 mg are presented in Table 1.
Study II evaluated 89 patients and had similar inclusion criteria to Study I except that subjects in Study II had additionally received MTX for at least 6 months with a stable dose (12.5 to 25 mg/week) for at least 4 weeks and they had at least 6 tender or painful joints. Subjects in Study II received a dose of 25 mg ENBREL® or placebo SC twice a week for 6 months in addition to their stable MTX dose.
Study III compared the efficacy of ENBREL® to MTX in patients with active RA. This study evaluated 632 patients who were ≥ 18 years old with early (≤ 3 years disease duration) active RA; had never received treatment with MTX; and had ≥ 12 tender joints, ≥ 10 swollen joints, and either ESR ≥ 28 mm/hr, CRP > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg ENBREL® were administered SC twice a week for 12 consecutive months. The study was unblinded after all patients had completed at least 12 months (and a median of 17.3 months) of therapy. The majority of patients remained in the study on the treatment to which they were randomized through 2 years, after which they entered an extension study and received open-label 25 mg ENBREL®. Results from patients receiving 25 mg are presented in Table 1. MTX tablets (escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial) or placebo tablets were given once a week on the same day as the injection of placebo or ENBREL® doses, respectively.
Study IV evaluated 682 adult patients with active RA of 6 months to 20 years duration (mean of 7 years) who had an inadequate response to at least one DMARD other than MTX. Forty-three percent of patients had previously received MTX a mean of two years prior to the trial at a mean dose of 12.9 mg. Patients were excluded from this study if MTX had been discontinued for lack of efficacy or for safety considerations. The patient baseline characteristics were similar to those of patients in Study I (Table 3). Patients were randomized to MTX alone (7.5 to 20 mg weekly, dose escalated as described for Study III; median dose 20 mg), ENBREL® alone (25 mg twice weekly), or the combination of ENBREL® and MTX initiated concurrently (at the same doses as above). The study evaluated ACR response, Sharp radiographic score and safety.
Clinical Response
A higher percentage of patients treated with ENBREL® and ENBREL® in combination with MTX achieved ACR 20, ACR 50, and ACR 70 responses and Major Clinical Responses than in the comparison groups. The results of Studies I, II, and III are summarized in Table 1. The results of Study IV are summarized in Table 2.
| Response | Placebo Controlled | Active Controlled | ||||
| Study I | Study II | Study III | ||||
| Placebo N = 80 | ENBREL®*
N = 78 | MTX/Placebo N = 30 | MTX/ENBREL®*
N = 59 | MTX N = 217 | ENBREL®*
N = 207 |
|
| ACR 20 | ||||||
| Month 3 | 23% | 62%† | 33% | 66%† | 56% | 62% |
| Month 6 | 11% | 59%† | 27% | 71%† | 58% | 65% |
| Month 12 | NA | NA | NA | NA | 65% | 72% |
| ACR 50 | ||||||
| Month 3 | 8% | 41%† | 0% | 42%† | 24% | 29% |
| Month 6 | 5% | 40%† | 3% | 39%† | 32% | 40% |
| Month 12 | NA | NA | NA | NA | 43% | 49% |
| ACR 70 | ||||||
| Month 3 | 4% | 15%† | 0% | 15%† | 7% | 13%‡ |
| Month 6 | 1% | 15%† | 0% | 15%† | 14% | 21%‡ |
| Month 12 | NA | NA | NA | NA | 22% | 25% |
| Endpoint | MTX (N = 228) | ENBREL® (N = 223) | ENBREL®/MTX (N = 231) |
| ACR N*† | |||
| Month 12 | 40 | 47 | 63‡ |
| ACR 20 | |||
| Month 12 | 59% | 66% | 75%‡ |
| ACR 50 | |||
| Month 12 | 36% | 43% | 63%‡ |
| ACR 70 | |||
| Month 12 | 17% | 22% | 40%‡ |
| Major Clinical Response§ | 6% | 10% | 24%‡ |
The time course for ACR 20 response rates for patients receiving placebo or 25 mg ENBREL® in Studies I and II is summarized in Figure 1. The time course of responses to ENBREL® in Study III was similar.
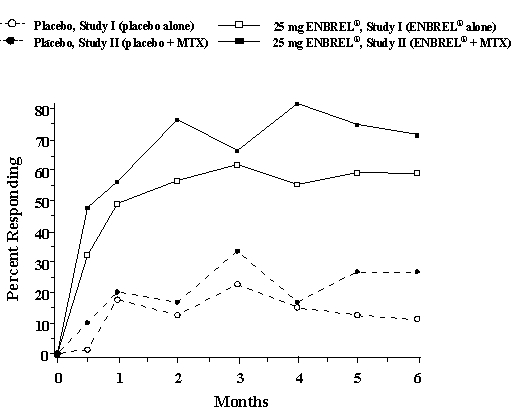
Figure 1: Time Course of ACR 20 Responses
Among patients receiving ENBREL®, the clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always occurred by 3 months. A dose response was seen in Studies I and III: 25 mg ENBREL® was more effective than 10 mg (10 mg was not evaluated in Study II). ENBREL® was significantly better than placebo in all components of the ACR criteria as well as other measures of RA disease activity not included in the ACR response criteria, such as morning stiffness.
In Study III, ACR response rates and improvement in all the individual ACR response criteria were maintained through 24 months of ENBREL® therapy. Over the 2-year study, 23% of ENBREL® patients achieved a major clinical response, defined as maintenance of an ACR 70 response over a 6-month period.
The results of the components of the ACR response criteria for Study I are shown in Table 3. Similar results were observed for ENBREL®-treated patients in Studies II and III.
|
||||
|
Placebo |
ENBREL®*
|
|||
| Parameter (median) | Baseline | 3 Months | Baseline | 3 Months† |
| Number of tender joints‡ | 34.0 | 29.5 | 31.2 | 10.0§ |
| Number of swollen joints¶ | 24.0 | 22.0 | 23.5 | 12.6§ |
| Physician global assessment# | 7.0 | 6.5 | 7.0 | 3.0§ |
| Patient global assessment# | 7.0 | 7.0 | 7.0 | 3.0§ |
| Pain# | 6.9 | 6.6 | 6.9 | 2.4§ |
| Disability indexÞ | 1.7 | 1.8 | 1.6 | 1.0§ |
| ESR (mm/hr) | 31.0 | 32.0 | 28.0 | 15.5§ |
| CRP (mg/dL) | 2.8 | 3.9 | 3.5 | 0.9§ |
After discontinuation of ENBREL®, symptoms of arthritis generally returned within a month. Reintroduction of treatment with ENBREL® after discontinuations of up to 18 months resulted in the same magnitudes of response as patients who received ENBREL® without interruption of therapy based on results of open-label studies.
Continued durable responses were seen for over 60 months in open-label extension treatment trials when patients received ENBREL® without interruption. A substantial number of patients who initially received concomitant MTX or corticosteroids were able to reduce their doses or discontinue these concomitant therapies while maintaining their clinical responses.
A 24-week study was conducted in 242 patients with active RA on background methotrexate who were randomized to receive either ENBREL® alone or the combination of ENBREL® and anakinra. The ACR 50 response rate was 31% for patients treated with the combination of ENBREL® and anakinra and 41% for patients treated with ENBREL® alone, indicating no added clinical benefit of the combination over ENBREL® alone. Serious infections were increased with the combination compared to ENBREL® alone (see WARNINGS).
Physical Function Response
In Studies I, II, and III, physical function and disability were assessed using the Health Assessment Questionnaire (HAQ).1 Additionally, in Study III, patients were administered the SF-362 Health Survey. In Studies I and II, patients treated with 25 mg ENBREL® twice weekly showed greater improvement from baseline in the HAQ score beginning in month 1 through month 6 in comparison to placebo (p < 0.001) for the HAQ disability domain (where 0 = none and 3 = severe). In Study I, the mean improvement in the HAQ score from baseline to month 6 was 0.6 (from 1.6 to 1.0) for the 25 mg ENBREL® group and 0 (from 1.7 to 1.7) for the placebo group. In Study II, the mean improvement from baseline to month 6 was 0.6 (from 1.5 to 0.9) for the ENBREL®/MTX group and 0.2 (from 1.3 to 1.2) for the placebo/MTX group. In Study III, the mean improvement in the HAQ score from baseline to month 6 was 0.7 (from 1.5 to 0.7) for 25 mg ENBREL® twice weekly. All subdomains of the HAQ in Studies I and III were improved in patients treated with ENBREL®.
In Study III, patients treated with 25 mg ENBREL® twice weekly showed greater improvement from baseline in SF-36 physical component summary score compared to ENBREL® 10 mg twice weekly and no worsening in the SF-36 mental component summary score. In open-label ENBREL® studies, improvements in physical function and disability measures have been maintained for up to 4 years.
In Study IV, median HAQ scores improved from baseline levels of 1.8, 1.8, and 1.8 to 1.1, 1.0, and 0.6 at 12 months in the MTX, ENBREL®, and ENBREL®/MTX combination treatment groups, respectively (combination versus both MTX and ENBREL®, p < 0.01). Twenty-nine percent of patients in the MTX alone treatment group had an improvement of HAQ of at least one unit versus 40% and 51% in the ENBREL® alone and the ENBREL®/MTX combination treatment groups, respectively.
Radiographic Response
In Study III, structural joint damage was assessed radiographically and expressed as change in total Sharp score (TSS) and its components, the erosion score and joint space narrowing (JSN) score. Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months, 12 months, and 24 months and scored by readers who were unaware of treatment group. The results are shown in Table 4. A significant difference for change in erosion score was observed at 6 months and maintained at 12 months.
|
|||||
| MTX | 25 mg ENBREL® |
MTX/ENBREL® (95% Confidence Interval*) | P-value | ||
| 12 Months | Total Sharp score | 1.59 | 1.00 | 0.59 (-0.12, 1.30) | 0.1 |
| Erosion score | 1.03 | 0.47 | 0.56 (0.11, 1.00) | 0.002 | |
| JSN score | 0.56 | 0.52 | 0.04 (-0.39, 0.46) | 0.5 | |
| 6 Months | Total Sharp score | 1.06 | 0.57 | 0.49 (0.06, 0.91) | 0.001 |
| Erosion score | 0.68 | 0.30 | 0.38 (0.09, 0.66) | 0.001 | |
| JSN score | 0.38 | 0.27 | 0.11 (-0.14, 0.35) | 0.6 | |
Patients continued on the therapy to which they were randomized for the second year of Study III. Seventy-two percent of patients had x-rays obtained at 24 months. Compared to the patients in the MTX group, greater inhibition of progression in TSS and erosion score was seen in the 25 mg ENBREL® group, and in addition, less progression was noted in the JSN score.
In the open-label extension of Study III, 48% of the original patients treated with 25 mg ENBREL® have been evaluated radiographically at 5 years. Patients had continued inhibition of structural damage, as measured by the TSS, and 55% of them had no progression of structural damage. Patients originally treated with MTX had further reduction in radiographic progression once they began treatment with ENBREL®.
In Study IV, less radiographic progression (TSS) was observed with ENBREL® in combination with MTX compared with ENBREL® alone or MTX alone at month 12 (Table 5). In the MTX treatment group 55% of patients experienced no radiographic progression (TSS change ≤ 0.0) at 12 months compared to 63% and 76% in the ENBREL® alone and the ENBREL®/MTX combination treatment groups, respectively.
|
MTX (N = 212)* |
ENBREL® (N = 212)* |
ENBREL®/MTX (N = 218)* |
|
| Total Sharp Scores (TSS) | 2.80 (1.08, 4.51) | 0.52† (-0.10, 1.15) | -0.54‡§ (-1.00, -0.07) |
| Erosion Score (ES) |
1.68 (0.61, 2.74) |
0.21† (-0.20, 0.61) |
-0.30‡ (-0.65, 0.04) |
| Joint Space Narrowing Score (JSN) | 1.12 (0.34, 1.90) |
0.32 (0.00, 0.63) | -0.23ठ(-0.45, -0.02) |
Once Weekly Dosing
The safety and efficacy of 50 mg ENBREL® (two 25 mg SC injections) administered once weekly were evaluated in a double-blind, placebo-controlled study of 420 patients with active RA. Fifty-three patients received placebo, 214 patients received 50 mg ENBREL® once weekly, and 153 patients received 25 mg ENBREL® twice weekly. The safety and efficacy profiles of the two ENBREL® treatment groups were similar.
Polyarticular Course Juvenile Idiopathic Arthritis (JIA)
The safety and efficacy of ENBREL® were assessed in a two-part study in 69 children with polyarticular-course JIA who had a variety of JIA onset types. Patients ages 4 to 17 years with moderately to severely active polyarticular-course JIA refractory to or intolerant of methotrexate were enrolled; patients remained on a stable dose of a single nonsteroidal anti-inflammatory drug and/or prednisone (≤ 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients received 0.4 mg/kg (maximum 25 mg per dose) ENBREL® SC twice weekly. In part 2, patients with a clinical response at day 90 were randomized to remain on ENBREL® or receive placebo for four months and assessed for disease flare. Responses were measured using the JIA Definition of Improvement (DOI),3 defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no more than one of the six JIA core set criteria, including active joint count, limitation of motion, physician and patient/parent global assessments, functional assessment, and ESR. Disease flare was defined as a ≥ 30% worsening in three of the six JIA core set criteria and ≥ 30% improvement in not more than one of the six JIA core set criteria and a minimum of two active joints.
In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part 2, 6 of 25 (24%) patients remaining on ENBREL® experienced a disease flare compared to 20 of 26 (77%) patients receiving placebo (p = 0.007). From the start of part 2, the median time to flare was ≥ 116 days for patients who received ENBREL® and 28 days for patients who received placebo. Each component of the JIA core set criteria worsened in the arm that received placebo and remained stable or improved in the arm that continued on ENBREL®. The data suggested the possibility of a higher flare rate among those patients with a higher baseline ESR. Of patients who demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients remaining on ENBREL® continued to improve from month 3 through month 7, while those who received placebo did not improve.
The majority of JIA patients who developed a disease flare in part 2 and reintroduced ENBREL® treatment up to 4 months after discontinuation re-responded to ENBREL® therapy in open-label studies. Most of the responding patients who continued ENBREL® therapy without interruption have maintained responses for up to 48 months.
Studies have not been done in patients with polyarticular-course JIA to assess the effects of continued ENBREL® therapy in patients who do not respond within 3 months of initiating ENBREL® therapy, or to assess the combination of ENBREL® with methotrexate.
Psoriatic Arthritis
The safety and efficacy of ENBREL® were assessed in a randomized, double-blind, placebo-controlled study in 205 patients with psoriatic arthritis. Patients were between 18 and 70 years of age and had active psoriatic arthritis (≥ 3 swollen joints and ≥ 3 tender joints) in one or more of the following forms: (1) distal interphalangeal (DIP) involvement (N = 104); (2) polyarticular arthritis (absence of rheumatoid nodules and presence of psoriasis; N = 173); (3) arthritis mutilans (N = 3); (4) asymmetric psoriatic arthritis (N = 81); or (5) ankylosing spondylitis-like (N = 7). Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients on MTX therapy at enrollment (stable for ≥ 2 months) could continue at a stable dose of ≤ 25 mg/week MTX. Doses of 25 mg ENBREL® or placebo were administered SC twice a week during the initial 6-month double-blind period of the study. Patients continued to receive blinded therapy in an up to 6-month maintenance period until all patients had completed the controlled period. Following this, patients received open-label 25 mg ENBREL® twice a week in a 12-month extension period.
Compared to placebo, treatment with ENBREL® resulted in significant improvements in measures of disease activity (Table 6).
|
||||
|
Placebo N = 104 |
ENBREL®* N = 101 |
|||
| Parameter (median) | Baseline | 6 Months | Baseline | 6 Months |
| Number of tender joints† | 17.0 | 13.0 | 18.0 | 5.0 |
| Number of swollen joints‡ | 12.5 | 9.5 | 13.0 | 5.0 |
| Physician global assessment§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Patient global assessment§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Morning stiffness (minutes) | 60 | 60 | 60 | 15 |
| Pain§ | 3.0 | 3.0 | 3.0 | 1.0 |
| Disability index¶ | 1.0 | 0.9 | 1.1 | 0.3 |
| CRP (mg/dL)# | 1.1 | 1.1 | 1.6 | 0.2 |
Among patients with psoriatic arthritis who received ENBREL®, the clinical responses were apparent at the time of the first visit (4 weeks) and were maintained through 6 months of therapy. Responses were similar in patients who were or were not receiving concomitant methotrexate therapy at baseline. At 6 months, the ACR 20/50/70 responses were achieved by 50%, 37%, and 9%, respectively, of patients receiving ENBREL®, compared to 13%, 4%, and 1%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of psoriatic arthritis, although few patients were enrolled with the arthritis mutilans and ankylosing spondylitis-like subtypes. The results of this study were similar to those seen in an earlier single-center, randomized, placebo-controlled study of 60 patients with psoriatic arthritis.
The skin lesions of psoriasis were also improved with ENBREL®, relative to placebo, as measured by percentages of patients achieving improvements in the Psoriasis Area and Severity Index (PASI).4 Responses increased over time, and at 6 months, the proportions of patients achieving a 50% or 75% improvement in the PASI were 47% and 23%, respectively, in the ENBREL® group (N = 66), compared to 18% and 3%, respectively, in the placebo group (N = 62). Responses were similar in patients who were or were not receiving concomitant methotrexate therapy at baseline.
Radiographic Response
Radiographic changes were also assessed in the psoriatic arthritis study. Radiographs of hands and wrists were obtained at baseline and months 6, 12, and 24. A modified Total Sharp Score (TSS), which included distal interphalangeal joints (i.e., not identical to the modified TSS used for rheumatoid arthritis) was used by readers blinded to treatment group to assess the radiographs. Some radiographic features specific to psoriatic arthritis (e.g., pencil-and-cup deformity, joint space widening, gross osteolysis and ankylosis) were included in the scoring system, but others (e.g., phalangeal tuft resorption, juxta-articular and shaft periostitis) were not.
Most patients showed little or no change in the modified TSS during this 24-month study (median change of 0 in both patients who initially received ENBREL® or placebo). More placebo-treated patients experienced larger magnitudes of radiographic worsening (increased TSS) compared to ENBREL® treatment during the controlled period of the study. At 12 months, in an exploratory analysis, 12% (12 of 104) of placebo patients compared to none of the 101 ENBREL®-treated patients had increases of 3 points or more in TSS. Inhibition of radiographic progression was maintained in patients who continued on ENBREL® during the second year. Of the patients with one-year and two-year x-rays, 3% (2 of 71) had increases of 3 points or more in TSS at one and two years.
Physical Function Response
In the psoriatic arthritis study, physical function and disability were assessed using the HAQ Disability Index (HAQ-DI)1 and the SF-362 Health Survey. Patients treated with 25 mg ENBREL® twice weekly showed greater improvement from baseline in the HAQ-DI score (mean decreases of 54% at both months 3 and 6) in comparison to placebo (mean decreases of 6% at both months 3 and 6) (p < 0.001). At months 3 and 6, patients treated with ENBREL® showed greater improvement from baseline in the SF-36 physical component summary score compared to patients treated with placebo, and no worsening in the SF-36 mental component summary score. Improvements in physical function and disability measures were maintained for up to 2 years through the open-label portion of the study.
Ankylosing Spondylitis
The safety and efficacy of ENBREL® were assessed in a randomized, double-blind, placebo-controlled study in 277 patients with active ankylosing spondylitis. Patients were between 18 and 70 years of age and had ankylosing spondylitis as defined by the modified New York Criteria for Ankylosing Spondylitis.5 Patients were to have evidence of active disease based on values of ≥ 30 on a 0 – 100 unit Visual Analog Scale (VAS) for the average of morning stiffness duration and intensity, and 2 of the following 3 other parameters: a) patient global assessment, b) average of nocturnal and total back pain, and c) the average score on the Bath Ankylosing Spondylitis Functional Index (BASFI). Patients with complete ankylosis of the spine were excluded from study participation. Patients taking hydroxychloroquine, sulfasalazine, methotrexate, or prednisone (≤ 10 mg/day) could continue these drugs at stable doses for the duration of the study. Doses of 25 mg ENBREL® or placebo were administered SC twice a week for 6 months.
The primary measure of efficacy was a 20% improvement in the Assessment in Ankylosing Spondylitis (ASAS) response criteria.6 Compared to placebo, treatment with ENBREL® resulted in improvements in the ASAS and other measures of disease activity (Figure 2 and Table 7).
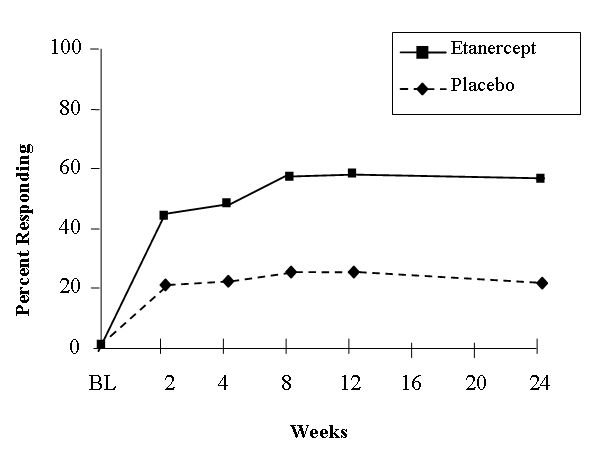
Figure 2: ASAS 20 Responses in Ankylosing Spondylitis
At 12 weeks, the ASAS 20/50/70 responses were achieved by 60%, 45%, and 29%, respectively, of patients receiving ENBREL®, compared to 27%, 13%, and 7%, respectively, of patients receiving placebo (p ≤ 0.0001, ENBREL® vs. placebo). Similar responses were seen at week 24. Responses were similar between those patients receiving concomitant therapies at baseline and those who were not. The results of this study were similar to those seen in a single-center, randomized, placebo-controlled study of 40 patients and a multi-center, randomized, placebo-controlled study of 84 patients with ankylosing spondylitis.
|
||||
|
Placebo N = 139 |
ENBREL®* N = 138 |
|||
| Mean values at time points | Baseline | 6 Months | Baseline | 6 Months |
| ASAS response criteria | ||||
| Patient global assessment † | 63 | 56 | 63 | 36 |
| Back pain‡ | 62 | 56 | 60 | 34 |
| BASFI§ | 56 | 55 | 52 | 36 |
| Inflammation¶ | 64 | 57 | 61 | 33 |
| Acute phase reactants | ||||
| CRP (mg/dL)# | 2.0 | 1.9 | 1.9 | 0.6 |
| Spinal mobility (cm): | ||||
| Modified Schober’s test | 3.0 | 2.9 | 3.1 | 3.3 |
| Chest expansion | 3.2 | 3.0 | 3.3 | 3.9 |
| Occiput-to-wall measurement | 5.3 | 6.0 | 5.6 | 4.5 |
Plaque Psoriasis
The safety and efficacy of ENBREL® were assessed in two randomized, double-blind, placebo-controlled studies in adults with chronic stable plaque psoriasis involving ≥ 10% of the body surface area, a minimum PASI of 10 and who had received or were candidates for systemic anti-psoriatic therapy or phototherapy. Patients with guttate, erythrodermic, or pustular psoriasis and patients with severe infections within 4 weeks of screening were excluded from study. No concomitant major anti-psoriatic therapies were allowed during the study.
Study I evaluated 672 patients who received placebo or ENBREL® SC at doses of 25 mg once a week, 25 mg twice a week or 50 mg twice a week for 3 months. After 3 months, patients continued on blinded treatments for an additional 3 months during which time, patients originally randomized to placebo began treatment with blinded ENBREL® at 25 mg twice weekly (designated as placebo/ENBREL® in Table 8); patients originally randomized to ENBREL® continued on the originally randomized dose (designated as ENBREL®/ENBREL® groups in Table 8).
Study II evaluated 611 patients who received placebo or ENBREL® SC at doses of 25 mg or 50 mg twice a week for 3 months. After 3 months of randomized blinded treatment, patients in all three arms began receiving open-label ENBREL® at 25 mg twice weekly for 9 additional months.
Response to treatment in both studies was assessed after 3 months of therapy and was defined as the proportion of patients who achieved a reduction in score of at least 75% from baseline by the Psoriasis Area and Severity Index (PASI). The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema, and scaling).
Other evaluated outcomes included the proportion of patients who achieved a score of “clear” or “minimal” by the Static Physician Global Assessment (sPGA) and the proportion of patients with a reduction of PASI of at least 50% from baseline. The sPGA is a 6 category scale ranging from “5 = severe” to “0 = none” indicating the physician’s overall assessment of the psoriasis severity focusing on induration, erythema, and scaling. Treatment success of “clear” or “minimal” consisted of none or minimal elevation in plaque, up to faint red coloration in erythema, and none or minimal fine scale over < 5% of the plaque.
Patients in all treatment groups and in both studies had a median baseline PASI score ranging from 15 to 17; and the percentage of patients with baseline sPGA classifications ranged from 54% to 66% for moderate, 17% to 26% for marked, and 1% to 5% for severe. Across all treatment groups, the percentage of patients who previously received systemic therapy for psoriasis ranged from 61% to 65% in Study I, and 71% to 75% in Study II; and those who previously received phototherapy ranged from 44% to 50% in Study I, and 72% to 73% in Study II.
More patients randomized to ENBREL® than placebo achieved at least a 75% reduction from baseline PASI score (PASI 75) with a dose response relationship across doses of 25 mg once a week, 25 mg twice a week and 50 mg twice a week (Table 8 and 9). The individual components of the PASI (induration, erythema, and scaling) contributed comparably to the overall treatment-associated improvement in PASI.
| ENBREL®/ENBREL® | ||||
| Placebo/ENBREL® 25 mg BIW | 25 mg QW | 25 mg BIW | 50 mg BIW | |
| (N = 168) | (N = 169) | (N = 167) | (N = 168) | |
| 3 Months | ||||
| PASI 75 n (%) | 6 (4%) | 23 (14%)* | 53 (32%)† | 79 (47%)† |
| Difference (95% CI) | 10% (4, 16) | 28% (21, 36) | 43% (35, 52) | |
| sPGA, “clear” or “minimal” n (%) | 8 (5%) | 36 (21%)† | 53 (32%)† | 79 (47%)† |
| Difference (95% CI) | 17% (10, 24) | 27% (19, 35) | 42% (34, 50) | |
| PASI 50 n (%) | 24 (14%) | 62 (37%)† | 90 (54%)† | 119 (71%)† |
| Difference (95% CI) | 22% (13, 31) | 40% (30, 49) | 57% (48, 65) | |
| 6 Months | ||||
| PASI 75 n (%) | 55 (33%) | 36 (21%) | 68 (41%) | 90 (54%) |
|
|||
| ENBREL® | |||
| Placebo | 25 mg BIW | 50 mg BIW | |
| (N = 204) | (N = 204) | (N = 203) | |
| PASI 75 n (%) | 6 (3%) | 66 (32%)* | 94 (46%)* |
| Difference (95% CI) | 29% (23, 36) | 43% (36, 51) | |
| sPGA “clear” or “minimal” n (%) | 7 (3%) | 75 (37%)* | 109 (54%)* |
| Difference (95% CI) | 34% (26, 41) | 50% (43, 58) | |
| PASI 50 n (%) | 18 (9%) | 124 (61%)* | 147 (72%)* |
| Difference (95% CI) | 52% (44, 60) | 64% (56, 71) | |
Among PASI 75 achievers in both studies, the median time to PASI 50 and PASI 75 was approximately 1 and approximately 2 months, respectively, after the start of therapy with either 25 or 50 mg twice a week.
In Study I patients who achieved PASI 75 at month 6 were entered into a study drug withdrawal and retreatment period. Following withdrawal of study drug, these patients had a median duration of PASI 75 of between 1 and 2 months.
In Study I, in patients who were PASI 75 responders at 3 months, retreatment with open-label ENBREL® after discontinuation of up to 5 months resulted in a similar proportion of responders as was seen during the initial double-blind portion of the study.
In Study II, most patients initially randomized to 50 mg twice a week continued in the study after month 3 and had their ENBREL® dose decreased to 25 mg twice a week. Of the 91 patients who were PASI 75 responders at month 3, 70 (77%) maintained their PASI 75 response at month 6.
INDICATIONS AND USAGE
ENBREL® is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis. ENBREL® can be initiated in combination with methotrexate (MTX) or used alone.
ENBREL® is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis in patients ages 2 and older.
ENBREL® is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in patients with psoriatic arthritis. ENBREL® can be used in combination with methotrexate in patients who do not respond adequately to methotrexate alone.
ENBREL® is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis.
ENBREL® is indicated for the treatment of adult patients (18 years or older) with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
CONTRAINDICATIONS
ENBREL® should not be administered to patients with sepsis or with known hypersensitivity to ENBREL® or any of its components.
WARNINGS
Risk of Serious Infections
(see also Boxed Warning)
Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in patients receiving TNF-blocking agents. Among opportunistic infections, tuberculosis, histoplasmosis, aspergillosis, candidiasis, coccidioidomycosis, listeriosis, and pneumocystosis were the most commonly reported. Patients have frequently presented with disseminated rather than localized disease, and are often taking concomitant immunosuppressants such as methotrexate or corticosteroids with ENBREL®.
Treatment with ENBREL® should not be initiated in patients with an active infection, including clinically important localized infections. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection;
- Who have been exposed to tuberculosis;
- Who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or
- With underlying conditions that may predispose them to infection such as advanced or poorly controlled diabetes (see PRECAUTIONS and ADVERSE REACTIONS: Infections).
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving ENBREL®, including patients who have previously received treatment for latent or active tuberculosis. Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent tuberculosis infection is lower with ENBREL® than with TNF-blocking monoclonal antibodies. Nonetheless, post-marketing cases of tuberculosis reactivation have been reported for TNF blockers, including ENBREL®. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating ENBREL® and periodically during therapy.
Treatment of latent tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating ENBREL®, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
Anti-tuberculosis therapy should also be considered prior to initiation of ENBREL® in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Tuberculosis should be strongly considered in patients who develop a new infection during ENBREL® treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with ENBREL®, including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may be falsely negative while on therapy with ENBREL®.
ENBREL® should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with ENBREL® should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
In 38 ENBREL® clinical trials and 4 cohort studies in all approved indications representing 27,169 patient years of exposure (17,696 patients) from the United States and Canada, no histoplasmosis infections were reported among patients treated with ENBREL®. Nonetheless, post marketing cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF blockers, including ENBREL®. For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of antifungal therapy.
In a 24-week study of concurrent ENBREL® and anakinra therapy, the rate of serious infections in the combination arm (7%) was higher than with ENBREL® alone (0%). The combination of ENBREL® and anakinra did not result in higher ACR response rates compared to ENBREL® alone (see CLINICAL STUDIES: Clinical Response and ADVERSE REACTIONS: Infections). Concurrent therapy with ENBREL® and anakinra is not recommended.
Neurologic Events
Treatment with ENBREL® and other agents that inhibit TNF have been associated with rare cases of new onset or exacerbation of central nervous system demyelinating disorders, some presenting with mental status changes and some associated with permanent disability. Cases of transverse myelitis, optic neuritis, multiple sclerosis, and new onset or exacerbation of seizure disorders have been observed in association with ENBREL® therapy. The causal relationship to ENBREL® therapy remains unclear. While no clinical trials have been performed evaluating ENBREL® therapy in patients with multiple sclerosis, other TNF antagonists administered to patients with multiple sclerosis have been associated with increases in disease activity.7, 8 Prescribers should exercise caution in considering the use of ENBREL® in patients with preexisting or recent-onset central nervous system demyelinating disorders (see ADVERSE REACTIONS).
Hematologic Events
Rare reports of pancytopenia including aplastic anemia, some with a fatal outcome, have been reported in patients treated with ENBREL®. The causal relationship to ENBREL® therapy remains unclear. Although no high risk group has been identified, caution should be exercised in patients being treated with ENBREL® who have a previous history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on ENBREL®. Discontinuation of ENBREL® therapy should be considered in patients with confirmed significant hematologic abnormalities.
Two percent of patients treated concurrently with ENBREL® and anakinra developed neutropenia (ANC < 1 x 109/L). While neutropenic, one patient developed cellulitis which recovered with antibiotic therapy.
Malignancies
In the controlled portions of clinical trials of all the TNF-blocking agents, more cases of lymphoma have been observed among patients receiving the TNF blocker compared to control patients. During the controlled portions of ENBREL® trials, 3 lymphomas were observed among 4509 ENBREL®-treated patients versus 0 among 2040 control patients (duration of controlled treatment ranged from 3 to 24 months). In the controlled and open-label portions of clinical trials of ENBREL®, 9 lymphomas were observed in 5723 patients over approximately 11,201 patient-years of therapy. This is 3-fold higher than that expected in the general population. While patients with rheumatoid arthritis or psoriasis, particularly those with highly active disease, may be at a higher risk (up to several fold) for the development of lymphoma, the potential role of TNF-blocking therapy in the development of malignancies is not known (see ADVERSE REACTIONS: Malignancies).11, 12
Cases of acute and chronic leukemia have been reported in association with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF-blocker therapy, patients with rheumatoid arthritis may be at higher risk (approximately 2-fold) than the general population for the development of leukemia.
During the controlled portions of ENBREL® trials, 2 cases of leukemia were observed among 5445 (0.06 cases per 100 patient years) ENBREL®-treated patients versus 0 among 2890 (0%) control patients (duration of controlled treatment ranged from 3 to 48 months).
Among 15,401 patients treated with ENBREL® in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of leukemia was 0.03 cases per 100 patient years.
Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including ENBREL® . Approximately half the cases were lymphomas, including Hodgkin’s and non-Hodgkin’s lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources including registries and spontaneous postmarketing reports.
In clinical trials of 696 patients, representing 1282 patient-years of therapy, no malignancies, including lymphoma or NMSC, have been reported.
Wegener’s Granulomatosis Patients
In a randomized, placebo-controlled study of 180 patients with Wegener's granulomatosis where ENBREL® was added to standard treatment (including cyclophosphamide, methotrexate, and corticosteroids), patients receiving ENBREL® experienced more non-cutaneous solid malignancies than patients receiving placebo (see ADVERSE REACTIONS: Malignancies). The addition of ENBREL® to standard treatment was not associated with improved clinical outcomes when compared with standard therapy alone. The use of ENBREL® in patients with Wegener’s granulomatosis receiving immunosuppressive agents is not recommended. The use of ENBREL® in patients receiving concurrent cyclophosphamide therapy is not recommended.
Hepatitis B Virus Reactivation
Use of TNF blockers, including ENBREL®, has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation. Patients at risk for HBV infection should be evaluated for prior evidence of HBV infection before initiating TNF blocker therapy. Prescribers should exercise caution in prescribing TNF blockers for patients identified as carriers of HBV. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with ENBREL® should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, consideration should be given to stopping ENBREL® and initiating anti-viral therapy with appropriate supportive treatment. The safety of resuming ENBREL® therapy after HBV reactivation is controlled is not known. Therefore, prescribers should weigh the risks and benefits when considering resumption of therapy in this situation.
Use in Patients with Moderate to Severe Alcoholic Hepatitis
In a study of 48 hospitalized patients treated with ENBREL® or placebo for moderate to severe alcoholic hepatitis, the mortality rate in patients treated with ENBREL® was similar to patients treated with placebo at one month but significantly higher after six months. Physicians should use caution when using ENBREL® in patients with moderate to severe alcoholic hepatitis.
PRECAUTIONS
General
Allergic reactions associated with administration of ENBREL® during clinical trials have been reported in < 2% of patients. If an anaphylactic reaction or other serious allergic reaction occurs, administration of ENBREL® should be discontinued immediately and appropriate therapy initiated.
Caution: The needle cap on the prefilled syringe and on the SureClick autoinjector contains dry natural rubber (a derivative of latex) which may cause allergic reactions in individuals sensitive to latex.
Information for patients
Patients or their caregivers should be provided the ENBREL® “Medication Guide” and provided an opportunity to read it and ask questions prior to initiation of therapy. The health care provider should ask the patient questions to determine any risk factors for treatment. Patients developing signs and symptoms of infection should seek medical evaluation immediately.
Latex Sensitivity Allergies
ENBREL® is provided as a single-use prefilled syringe, a single-use prefilled SureClick autoinjector, or a multiple-use vial. The patient or caregiver should be informed that the needle cap on the prefilled syringe and on the SureClick autoinjector contains dry natural rubber (a derivative of latex), which should not be handled by persons sensitive to latex.
Administration of ENBREL®
If a patient or caregiver is to administer ENBREL®, the patient or caregiver should be instructed in injection techniques and how to measure and administer the correct dose (see the ENBREL® (etanercept) “Patient Instructions for Use” insert). The first injection should be performed under the supervision of a qualified health care professional. The patient’s or caregiver’s ability to inject subcutaneously should be assessed. Patients and caregivers should be instructed in the technique as well as proper syringe and needle disposal, and be cautioned against reuse of needles and syringes. A puncture-resistant container for disposal of needles, syringes, and autoinjectors should be used. If the product is intended for multiple use, additional syringes, needles, and alcohol swabs will be required.
Patients with Heart Failure
Two large clinical trials evaluating the use of ENBREL® in the treatment of heart failure were terminated early due to lack of efficacy. Results of one study suggested higher mortality in patients treated with ENBREL® compared to placebo. Results of the second study did not corroborate these observations. Analyses did not identify specific factors associated with increased risk of adverse outcomes in heart failure patients treated with ENBREL® (see ADVERSE REACTIONS: Patients with Heart Failure). There have been post-marketing reports of worsening of congestive heart failure (CHF), with and without identifiable precipitating factors, in patients taking ENBREL®. There have also been rare reports of new onset CHF, including CHF in patients without known preexisting cardiovascular disease. Some of these patients have been under 50 years of age. Physicians should exercise caution when using ENBREL® in patients who also have heart failure, and monitor patients carefully.
Immunosuppression
Anti-TNF therapies, including ENBREL®, affect host defenses against infections and malignancies since TNF mediates inflammation and modulates cellular immune responses. In a study of 49 patients with RA treated with ENBREL®, there was no evidence of depression of delayed-type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector cell populations. The impact of treatment with ENBREL® on the development and course of malignancies, as well as active and/or chronic infections, is not fully understood (see WARNINGS: Malignancies, ADVERSE REACTIONS: Infections, andMalignancies). The safety and efficacy of ENBREL® in patients with immunosuppression or chronic infections have not been evaluated.
Immunizations
Most psoriatic arthritis patients receiving ENBREL® were able to mount effective B-cell immune responses to pneumococcal polysaccharide vaccine, but titers in aggregate were moderately lower and fewer patients had two-fold rises in titers compared to patients not receiving ENBREL®. The clinical significance of this is unknown. Patients receiving ENBREL® may receive concurrent vaccinations, except for live vaccines. No data are available on the secondary transmission of infection by live vaccines in patients receiving ENBREL® (see PRECAUTIONS: Immunosuppression).
It is recommended that JIA patients, if possible, be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating ENBREL® therapy. Patients with a significant exposure to varicella virus should temporarily discontinue ENBREL® therapy and be considered for prophylactic treatment with Varicella Zoster Immune Globulin.
Autoimmunity
Treatment with ENBREL® may result in the formation of autoantibodies (see ADVERSE REACTIONS: Autoantibodies) and, rarely, in the development of a lupus-like syndrome or autoimmune hepatitis (see ADVERSE REACTIONS: Adverse Reaction Information from Spontaneous Reports), which may resolve following withdrawal of ENBREL®. If a patient develops symptoms and findings suggestive of a lupus-like syndrome or autoimmune hepatitis following treatment with ENBREL®, treatment should be discontinued and the patient should be carefully evaluated.
Drug Interactions
Specific drug interaction studies have not been conducted with ENBREL®. However, it was observed that the pharmacokinetics of ENBREL® was unaltered by concomitant methotrexate in rheumatoid arthritis patients.
In a study in which patients with active RA were treated for up to 24 weeks with concurrent ENBREL® and anakinra therapy, a 7% rate of serious infections was observed, which was higher than that observed with ENBREL® alone (0%) (see also WARNINGS). Two percent of patients treated concurrently with ENBREL® and anakinra developed neutropenia (ANC < 1 x 109/L).
In a study of patients with Wegener’s granulomatosis, the addition of ENBREL® to standard therapy (including cyclophosphamide) was associated with a higher incidence of non-cutaneous solid malignancies. The use of ENBREL® in patients receiving concurrent cyclophosphamide therapy is not recommended (see WARNINGS: Malignancies and ADVERSE REACTIONS: Malignancies).
Patients in a clinical study who were on established therapy with sulfasalazine, to which ENBREL® was added, were noted to develop a mild decrease in mean neutrophil counts in comparison to groups treated with either ENBREL® or sulfasalazine alone. The clinical significance of this observation is unknown.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of ENBREL® or its effect on fertility. Mutagenesis studies were conducted in vitro and in vivo, and no evidence of mutagenic activity was observed.
Pregnancy (Category B)
Developmental toxicity studies have been performed in rats and rabbits at doses ranging from 60- to 100-fold higher than the human dose and have revealed no evidence of harm to the fetus due to ENBREL®. There are, however, no studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Registry: To monitor outcomes of pregnant women exposed to ENBREL®, a pregnancy registry has been established. Physicians are encouraged to register patients by calling 1-877-311-8972.
Nursing Mothers
It is not known whether ENBREL® is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ENBREL®, a decision should be made whether to discontinue nursing or to discontinue the drug.
Geriatric Use
A total of 480 RA patients ages 65 years or older have been studied in clinical trials. In plaque psoriasis randomized clinical trials, a total of 138 out of 1965 subjects treated with ENBREL® or placebo were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, but the number of geriatric psoriasis subjects is too small to determine whether they respond differently from younger subjects. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly.
Pediatric Use
ENBREL® is indicated for treatment of polyarticular-course juvenile idiopathic arthritis in patients ages 2 and older. For issues relevant to pediatric patients, in addition to other sections of the label, see also WARNINGS;PRECAUTIONS: Immunizations; and ADVERSE REACTIONS: Adverse Reactions in Patients with JIA. ENBREL® has not been studied in children < 2 years of age.
The safety and efficacy of ENBREL® in pediatric patients with plaque psoriasis have not been studied.
ADVERSE REACTIONS
Adverse Reactions in Adult Patients with RA, Psoriatic Arthritis, Ankylosing Spondylitis, or Plaque Psoriasis
ENBREL® has been studied in 1442 patients with RA, followed for up to 80 months, in 169 patients with psoriatic arthritis for up to 24 months, in 222 patients with ankylosing spondylitis for up to 10 months, and 1261 patients with plaque psoriasis for up to 15 months. In controlled trials, the proportion of ENBREL®-treated patients who discontinued treatment due to adverse events was approximately 4% in the indications studied. The vast majority of these patients were treated with 25 mg SC twice weekly. In plaque psoriasis studies, ENBREL® doses studied were 25 mg SC once a week, 25 mg SC twice a week, and 50 mg SC twice a week.
Injection Site Reactions
In controlled trials in rheumatologic indications, approximately 37% of patients treated with ENBREL® developed injection site reactions. In controlled trials in patients with plaque psoriasis, 14% of patients treated with ENBREL® developed injection site reactions during the first 3 months of treatment. All injection site reactions were described as mild to moderate (erythema and/or itching, pain, or swelling) and generally did not necessitate drug discontinuation. Injection site reactions generally occurred in the first month and subsequently decreased in frequency. The mean duration of injection site reactions was 3 to 5 days. Seven percent of patients experienced redness at a previous injection site when subsequent injections were given. In post-marketing experience, injection site bleeding and bruising have also been observed in conjunction with ENBREL® therapy.
Infections
In controlled trials, there were no differences in rates of infection among RA, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis patients treated with ENBREL® and those treated with placebo (or MTX for RA and psoriatic arthritis patients). The most common type of infection was upper respiratory infection, which occurred at a rate of approximately 20% among both ENBREL®- and placebo-treated patients in RA, psoriatic arthritis, and AS trials, and at a rate of approximately 12% among both ENBREL®- and placebo-treated patients in plaque psoriasis trials in the first 3 months of treatment.
In placebo-controlled trials in RA, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis no increase in the incidence of serious infections was observed (approximately 1% in both placebo- and ENBREL®-treated groups). In all clinical trials in RA, serious infections experienced by patients have included: pyelonephritis, bronchitis, septic arthritis, abdominal abscess, cellulitis, osteomyelitis, wound infection, pneumonia, foot abscess, leg ulcer, diarrhea, sinusitis, and sepsis. The rate of serious infections has not increased in open-label extension trials and is similar to that observed in ENBREL®- and placebo-treated patients from controlled trials. Serious infections, including sepsis and death, have also been reported during post-marketing use of ENBREL®. Some have occurred within a few weeks after initiating treatment with ENBREL®. Many of the patients had underlying conditions (e.g., diabetes, congestive heart failure, history of active or chronic infections) in addition to their rheumatoid arthritis (see WARNINGS). Data from a sepsis clinical trial not specifically in patients with RA suggest that ENBREL® treatment may increase mortality in patients with established sepsis.9
In patients who received both ENBREL® and anakinra for up to 24 weeks, the incidence of serious infections was 7%. The most common infections consisted of bacterial pneumonia (4 cases) and cellulitis (4 cases). One patient with pulmonary fibrosis and pneumonia died due to respiratory failure.
In post-marketing experience in rheumatologic indications, infections have been observed with various pathogens including viral, bacterial, fungal, and protozoal organisms. Infections have been noted in all organ systems and have been reported in patients receiving ENBREL® alone or in combination with immunosuppressive agents.
In clinical trials in plaque psoriasis, serious infections experienced by ENBREL®-treated patients have included: cellulitis, gastroenteritis, pneumonia, abscess, and osteomyelitis.
In global clinical studies of 20,070 patients (28,308 patient-years of therapy), tuberculosis was observed in approximately 0.01% of patients. In 15,438 patients (23,524 patient-years of therapy) from clinical studies in the US and Canada, tuberculosis was observed in approximately 0.007% of patients. These studies include reports of pulmonary and extra-pulmonary tuberculosis (see WARNINGS).
Malignancies
Patients have been observed in clinical trials with ENBREL® for over five years. Among 4462 rheumatoid arthritis patients treated with ENBREL® in clinical trials for a mean of 27 months (approximately 10000 patient-years of therapy), 9 lymphomas were observed for a rate of 0.09 cases per 100 patient-years. This is 3-fold higher than the rate of lymphomas expected in the general population based on the Surveillance, Epidemiology, and End Results Database.10 An increased rate of lymphoma up to several fold has been reported in the rheumatoid arthritis patient population, and may be further increased in patients with more severe disease activity11, 12 (see WARNINGS: Malignancies). Sixty-seven malignancies, other than lymphoma, were observed. Of these, the most common malignancies were colon, breast, lung, and prostate, which were similar in type and number to what would be expected in the general population.10 Analysis of the cancer rates at 6 month intervals suggest constant rates over five years of observation.
In the placebo-controlled portions of the psoriasis studies, 8 of 933 patients who received ENBREL® at any dose were diagnosed with a malignancy compared to 1 of 414 patients who received placebo. Among the 1261 patients with psoriasis who received ENBREL® at any dose in the controlled and uncontrolled portions of the psoriasis studies (1062 patient-years), a total of 22 patients were diagnosed with 23 malignancies; 9 patients with non-cutaneous solid tumors, 12 patients with 13 non-melanoma skin cancers (8 basal, 5 squamous), and 1 patient with non-Hodgkin’s lymphoma. Among the placebo-treated patients (90 patient-years of observation) 1 patient was diagnosed with 2 squamous cell cancers. The size of the placebo group and limited duration of the controlled portions of studies precludes the ability to draw firm conclusions.
Among 89 patients with Wegener’s granulomatosis receiving ENBREL® in a randomized, placebo-controlled trial, 5 experienced a variety of non-cutaneous solid malignancies compared with none receiving placebo (see WARNINGS: Malignancies).
Immunogenicity
Patients with RA, psoriatic arthritis, ankylosing spondylitis, or plaque psoriasis were tested at multiple timepoints for antibodies to ENBREL®. Antibodies to the TNF receptor portion or other protein components of the ENBREL® drug product were detected at least once in sera of approximately 6% of adult patients with RA, psoriatic arthritis, ankylosing spondylitis, or plaque psoriasis. These antibodies were all non-neutralizing. Results from JIA patients were similar to those seen in adult RA patients treated with ENBREL®.
In PsO studies that evaluated the exposure of etanercept for up to 120 weeks, the percentage of patients testing positive at the assessed time points of 24, 48, 72, and 96 weeks ranged from 3.6%- 8.7% and were all non-neutralizing. The percentage of patients testing positive increased with an increase in the duration of study, however, the clinical significance of this finding is unknown. No apparent correlation of antibody development to clinical response or adverse events was observed. The immunogenicity data of ENBREL® beyond 120 weeks of exposure is unknown.
The data reflect the percentage of patients whose test results were considered positive for antibodies to ENBREL® in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of any antibody positivity in an assay is highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to ENBREL® with the incidence of antibodies to other products may be misleading.
Autoantibodies
Patients with RA had serum samples tested for autoantibodies at multiple timepoints. In RA Studies I and II, the percentage of patients evaluated for antinuclear antibodies (ANA) who developed new positive ANA (titer ≥ 1:40) was higher in patients treated with ENBREL® (11%) than in placebo-treated patients (5%). The percentage of patients who developed new positive anti-double-stranded DNA antibodies was also higher by radioimmunoassay (15% of patients treated with ENBREL® compared to 4% of placebo-treated patients) and by Crithidia luciliae assay (3% of patients treated with ENBREL® compared to none of placebo-treated patients). The proportion of patients treated with ENBREL® who developed anticardiolipin antibodies was similarly increased compared to placebo-treated patients. In Study III, no pattern of increased autoantibody development was seen in ENBREL® patients compared to MTX patients.
The impact of long-term treatment with ENBREL® on the development of autoimmune diseases is unknown. Rare adverse event reports have described patients with rheumatoid factor positive and/or erosive RA who have developed additional autoantibodies in conjunction with rash and other features suggesting a lupus-like syndrome.
Other Adverse Reactions
Table 10 summarizes events reported in at least 3% of all patients with higher incidence in patients treated with ENBREL® compared to controls in placebo-controlled RA trials (including the combination methotrexate trial) and relevant events from Study III. In placebo-controlled plaque psoriasis trials, the percentages of patients reporting injection site reactions were lower in the placebo dose group (6.4%) than in the ENBREL® dose groups (15.5%) in Studies I and II. Otherwise, the percentages of patients reporting adverse events in the 50 mg twice a week dose group were similar to those observed in the 25 mg twice a week dose group or placebo group. In psoriasis Study I, there were no serious adverse events of worsening psoriasis following withdrawal of study drug. However, adverse events of worsening psoriasis including three serious adverse events were observed during the course of the clinical trials. Urticaria and non-infectious hepatitis were observed in a small number of patients and angioedema was observed in one patient in clinical studies. Urticaria and angioedema have also been reported in spontaneous post-marketing reports. Adverse events in psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis trials were similar to those reported in RA clinical trials.
|
||||
|
Event | Placebo Controlled |
Active Controlled (Study III) |
||
| Percent of patients | Percent of patients | |||
|
Placebo† (N = 152) |
ENBREL® (N = 349) |
MTX (N = 217) |
ENBREL® (N = 415) |
|
| Injection site reaction | 10 | 37 | 7 | 34 |
| Infection (total)‡ | 32 | 35 | 72 | 64 |
| Non-upper respiratory infection (non-URI)‡ | 32 | 38 | 60 | 51 |
| Upper respiratory infection (URI)‡ | 16 | 29 | 39 | 31 |
| Headache | 13 | 17 | 27 | 24 |
| Nausea | 10 | 9 | 29 | 15 |
| Rhinitis | 8 | 12 | 14 | 16 |
| Dizziness | 5 | 7 | 11 | 8 |
| Pharyngitis | 5 | 7 | 9 | 6 |
| Cough | 3 | 6 | 6 | 5 |
| Asthenia | 3 | 5 | 12 | 11 |
| Abdominal pain | 3 | 5 | 10 | 10 |
| Rash | 3 | 5 | 23 | 14 |
| Peripheral edema | 3 | 2 | 4 | 8 |
| Respiratory disorder | 1 | 5 | NA | NA |
| Dyspepsia | 1 | 4 | 10 | 11 |
| Sinusitis | 2 | 3 | 3 | 5 |
| Vomiting | - | 3 | 8 | 5 |
| Mouth ulcer | 1 | 2 | 14 | 6 |
| Alopecia | 1 | 1 | 12 | 6 |
| Pneumonitis (“MTX lung”) | - | - | 2 | 0 |
In controlled trials of RA and psoriatic arthritis, rates of serious adverse events were seen at a frequency of approximately 5% among ENBREL®- and control-treated patients. In controlled trials of plaque psoriasis, rates of serious adverse events were seen at a frequency of < 1.5% among ENBREL®- and placebo-treated patients in the first 3 months of treatment. Among patients with RA in placebo-controlled, active-controlled, and open-label trials of ENBREL®, malignancies (see WARNINGS: Malignancies, ADVERSE REACTIONS: Malignancies) and infections (see ADVERSE REACTIONS: Infections) were the most common serious adverse events observed. Other infrequent serious adverse events observed in RA, psoriatic arthritis, ankylosing spondylitis, or plaque psoriasis clinical trials are listed by body system below:
| Cardiovascular: | heart failure, myocardial infarction, myocardial ischemia, hypertension, hypotension, deep vein thrombosis, thrombophlebitis |
| Digestive: | cholecystitis, pancreatitis, gastrointestinal hemorrhage, appendicitis |
| Hematologic/Lymphatic: | lymphadenopathy |
| Musculoskeletal: | bursitis, polymyositis |
| Nervous: | cerebral ischemia, depression, multiple sclerosis (see WARNINGS: Neurologic Events) |
| Respiratory: | dyspnea, pulmonary embolism, sarcoidosis |
| Skin: | worsening psoriasis |
| Urogenital: | membranous glomerulonephropathy, kidney calculus |
In a randomized controlled trial in which 51 patients with RA received ENBREL® 50 mg twice weekly and 25 patients received ENBREL® 25 mg twice weekly, the following serious adverse events were observed in the 50 mg twice weekly arm: gastrointestinal bleeding, normal pressure hydrocephalus, seizure, and stroke. No serious adverse events were observed in the 25 mg arm.
Adverse Reactions in Patients with JIA
In general, the adverse events in pediatric patients were similar in frequency and type as those seen in adult patients (see WARNINGS and other sections under ADVERSE REACTIONS). Differences from adults and other special considerations are discussed in the following paragraphs.
Severe adverse reactions reported in 69 JIA patients ages 4 to 17 years included varicella (see also PRECAUTIONS: Immunizations), gastroenteritis, depression/personality disorder, cutaneous ulcer, esophagitis/gastritis, group A streptococcal septic shock, Type 1 diabetes mellitus, and soft tissue and post-operative wound infection.
Forty-three of 69 (62%) children with JIA experienced an infection while receiving ENBREL® during three months of study (part 1 open-label), and the frequency and severity of infections was similar in 58 patients completing 12 months of open-label extension therapy. The types of infections reported in JIA patients were generally mild and consistent with those commonly seen in outpatient pediatric populations. Two JIA patients developed varicella infection and signs and symptoms of aseptic meningitis which resolved without sequelae.
The following adverse events were reported more commonly in 69 JIA patients receiving 3 months of ENBREL® compared to the 349 adult RA patients in placebo-controlled trials. These included headache (19% of patients, 1.7 events per patient-year), nausea (9%, 1.0 events per patient-year), abdominal pain (19%, 0.74 events per patient-year), and vomiting (13%, 0.74 events per patient-year).
In open-label clinical studies of children with JIA, adverse events reported in those aged 2 to 4 years were similar to adverse events reported in older children.
In post-marketing experience, the following additional serious adverse events have been reported in pediatric patients: abscess with bacteremia, optic neuritis, pancytopenia, seizures, tuberculous arthritis, urinary tract infection (see WARNINGS), coagulopathy, cutaneous vasculitis, and transaminase elevations. The frequency of these events and their causal relationship to ENBREL® therapy are unknown.
Patients with Heart Failure
Two randomized placebo-controlled studies have been performed in patients with CHF. In one study, patients received either ENBREL® 25 mg twice weekly, 25 mg three times weekly, or placebo. In a second study, patients received either ENBREL® 25 mg once weekly, 25 mg twice weekly, or placebo. Results of the first study suggested higher mortality in patients treated with ENBREL® at either schedule compared to placebo. Results of the second study did not corroborate these observations. Analyses did not identify specific factors associated with increased risk of adverse outcomes in heart failure patients treated with ENBREL® (see PRECAUTIONS: Patients with Heart Failure).
Adverse Reaction Information from Spontaneous Reports
Adverse events have been reported during post-approval use of ENBREL®. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to ENBREL® exposure.
Additional adverse events are listed by body system below:
| Body as a whole: | angioedema, fatigue, fever, flu syndrome, generalized pain, weight gain |
| Cardiovascular: | chest pain, vasodilation (flushing), new-onset congestive heart failure (see PRECAUTIONS: Patients with Heart Failure) |
| Digestive: | altered sense of taste, anorexia, diarrhea, dry mouth, intestinal perforation |
| Hematologic/Lymphatic: | adenopathy, anemia, aplastic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia (see WARNINGS) |
| Hepatobiliary: | autoimmune hepatitis |
| Musculoskeletal: | joint pain, lupus-like syndrome with manifestations including rash consistent with subacute or discoid lupus |
| Nervous: | paresthesias, stroke, seizures and central nervous system events suggestive of multiple sclerosis or isolated demyelinating conditions such as transverse myelitis or optic neuritis (see WARNINGS) |
| Ocular: | dry eyes, ocular inflammation |
| Respiratory: | dyspnea, interstitial lung disease, pulmonary disease, worsening of prior lung disorder |
| Skin: | cutaneous vasculitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, pruritus, subcutaneous nodules, urticaria, new or worsening psoriasis (all sub-types including pustular and palmoplantar) |
OVERDOSAGE
The maximum tolerated dose of ENBREL® has not been established in humans. Toxicology studies have been performed in monkeys at doses up to 30 times the human dose with no evidence of dose-limiting toxicities. No dose-limiting toxicities have been observed during clinical trials of ENBREL®. Single IV doses up to 60 mg/m2 have been administered to healthy volunteers in an endotoxemia study without evidence of dose-limiting toxicities.
DOSAGE AND ADMINISTRATION
General
A 50 mg dose should be given as one subcutaneous (SC) injection using either a 50 mg single-use prefilled syringe or a single-use prefilled SureClick autoinjector. A 50 mg dose can also be given as two 25 mg SC injections using 25 mg single-use prefilled syringes or multiple-use vials.
When administering ENBREL® as two injections in adults or children, the injections should be given either on the same day or 3 or 4 days apart (see CLINICAL STUDIES).
Sites for injection (thigh, abdomen, or upper arm) should be rotated. Never inject into areas where the skin is tender, bruised, red, or hard. See the ENBREL® (etanercept) “Patient Instructions for Use” insert for detailed information on injection site selection and dose administration.
Adult RA, AS, and Psoriatic Arthritis Patients
The recommended dose of ENBREL® for adult patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis is 50 mg per week. Methotrexate, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with ENBREL®. Based on a study of 50 mg ENBREL® twice weekly in patients with RA that suggested higher incidence of adverse reactions but similar ACR response rates, doses higher than 50 mg per week are not recommended (see ADVERSE REACTIONS).
Adult Plaque Psoriasis Patients
The recommended starting dose of ENBREL® for adult patients is a 50 mg dose given twice weekly (administered 3 or 4 days apart) for 3 months followed by a reduction to a maintenance dose of 50 mg per week (see CLINICAL STUDIES).
Starting doses of ENBREL® of 25 mg or 50 mg per week were also shown to be efficacious. The proportion of responders were related to ENBREL® dosage (see CLINICAL STUDIES).
JIA Patients
The recommended dose of ENBREL® for pediatric patients ages 2 to 17 years with active polyarticular-course JIA is 0.8 mg/kg per week (up to a maximum of 50 mg per week). The 25 mg prefilled syringe is not recommended for pediatric patients weighing less than 31 kg (68 pounds). The 50 mg prefilled syringe or SureClick autoinjector may be used for pediatric patients weighing 63 kg (138 pounds) or more. Glucocorticoids, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with ENBREL®. Concurrent use with methotrexate and higher doses of ENBREL® have not been studied in pediatric patients.
Preparation of ENBREL®
ENBREL® is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose.
The ENBREL® (etanercept) “Patient Instructions for Use” insert contains more detailed instructions on the preparation of ENBREL®.
Preparation of ENBREL® Using the Single-use Prefilled Syringe:
Before injection, ENBREL® may be allowed to reach room temperature (approximately 15 to 30 minutes). DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Prior to administration, visually inspect the solution for particulate matter and discoloration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present. Check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of ENBREL® Using the Single-use Prefilled SureClick Autoinjector:
Before injection, ENBREL® may be allowed to reach room temperature (approximately 15 to 30 minutes). DO NOT remove the needle shield while allowing the SureClick autoinjector to reach room temperature.
Prior to administration, visually inspect the solution for particulate matter and discoloration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present.
Preparation of ENBREL® Using the Multiple-use Vial:
ENBREL® should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol) giving a solution of 1.0 mL containing 25 mg of ENBREL®.
A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25-gauge needle should be used for reconstituting and withdrawing ENBREL®, and the supplied “Mixing Date:” sticker should be attached to the vial and the date of reconstitution entered. Reconstitution with the supplied BWFI, using a 25-gauge needle, yields a preserved, multiple-use solution that must be used within 14 days.
If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the ENBREL® vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the ENBREL® vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the ENBREL® vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
If using a 25-gauge needle to reconstitute and withdraw ENBREL®, the diluent should be injected very slowly into the ENBREL® vial. It is normal for some foaming to occur. The contents should be swirled gently during dissolution. To avoid excessive foaming, do not shake or vigorously agitate.
Generally, dissolution of ENBREL® takes less than 10 minutes. Visually inspect the solution for particulate matter and discoloration prior to administration. The solution should not be used if discolored or cloudy, or if particulate matter remains.
Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25-gauge needle from the syringe. Attach a 27-gauge needle to inject ENBREL®.
The contents of one vial of ENBREL® solution should not be mixed with, or transferred into, the contents of another vial of ENBREL®. No other medications should be added to solutions containing ENBREL®, and do not reconstitute ENBREL® with other diluents. Do not filter reconstituted solution during preparation or administration.
Reconstitution with the supplied BWFI, using a 25-gauge needle, yields a preserved, multiple-use solution that must be used within 14 days. Discard reconstituted solution after 14 days. PRODUCT STABILITY AND STERILITY CANNOT BE ASSURED AFTER 14 DAYS.
Storage and Stability
ENBREL® Single-use Prefilled Syringe and ENBREL® Single-use Prefilled SureClick Autoinjector:
Do not use ENBREL® beyond the expiration date stamped on the carton or barrel label. ENBREL® must be refrigerated at 2° to 8°C (36° to 46°F). DO NOT FREEZE. Keep the product in the original carton to protect from light until the time of use. Do not shake.
ENBREL® Multiple-use Vial:
Do not use a dose tray beyond the expiration date stamped on the carton, dose tray label, vial label, or diluent syringe label. The dose tray containing ENBREL® (sterile powder) must be refrigerated at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Reconstituted solutions of ENBREL® prepared with the supplied Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), using a 25-gauge needle, may be stored for up to 14 days if refrigerated at 2° to 8°C (36° to 46°F). Discard reconstituted solution after 14 days. PRODUCT STABILITY AND STERILITY CANNOT BE ASSURED AFTER 14 DAYS.
HOW SUPPLIED
Each ENBREL® single-useprefilled syringe and ENBREL® single-use prefilledSureClick autoinjector contains 50 mg/mL of etanercept in a single-dose syringe with a 27-gauge, ½-inch needle.
| 25 mg single-use prefilled syringe | Carton of 4 | NDC 58406-455-04 |
| 50 mg single-use prefilled syringe | Carton of 4 | NDC 58406-435-04 |
| 50 mg single-use prefilled SureClick autoinjector | Carton of 4 | NDC 58406-445-04 |
ENBREL® multiple-use vial is supplied in a carton containing four dose trays. Each dose tray contains one 25 mg vial of etanercept, one diluent syringe (1 mL Sterile Bacteriostatic Water for Injection, USP, containing 0.9% benzyl alcohol), one 27-gauge ½-inch needle, one vial adapter, one plunger, and two alcohol swabs. Each carton contains four “Mixing Date:" stickers.
| 25 mg multiple-use vial | Carton of 4 | NDC 58406-425-34 |
Administration of one 50 mg ENBREL® prefilled syringe or one ENBREL® SureClick autoinjector provides a dose equivalent to two 25 mg ENBREL® prefilled syringes or two multiple-use vials of lyophilized ENBREL®, when vials are reconstituted and administered as recommended.
REFERENCES
- Ramey DR, Fries JF, Singh G. The Health Assessment Questionnaire 1995 - Status and Review. In: Spilker B, ed. “Quality of Life and Pharmacoeconomics in Clinical Trials.” 2nd ed. Philadelphia, PA. Lippincott-Raven 1996;227.
- Ware JE Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998;51(11):903.
- Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement of juvenile arthritis. Arthritis Rheum 1997;40(7):1202.
- Fredriksson T, Petersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica 1978;157:238.
- van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27(4):361-8.
- Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum 2001;44(8):1876-86.
- Van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996;47:1531.
- Arnason BGW, et al. (Lenercept Multiple Sclerosis Study Group). TNF neutralization in MS: Results of a randomized, placebo-controlled multicenter study. Neurology 1999;53:457.
- Fisher CJ Jr, Agosti JM, Opal SM, et al. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med 1996;334(26):1697.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 11 Registries, 1992-1999.
- Mellemkjaer L, Linet MS, Gridley G, et al. Rheumatoid Arthritis and Cancer Risk. Eur J Cancer 1996;32A(10):1753-1757.
- Baecklund E, Ekbom A, Sparen P, et al. Disease Activity and Risk of Lymphoma in Patients With Rheumatoid Arthritis: Nested Case-Control Study. BMJ 1998;317:180-181.
[Amgen Logo]
[Wyeth Logo]
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
U.S. License Number 1132
Marketed by Amgen Inc. and Wyeth Pharmaceuticals
© 1998 – 2009 Immunex Corporation. All rights reserved.
1XXXXXX – v38
Issue Date: 11/2009
Patents covering methods, vectors, and/or host cells for making the product or methods for using the product:
U.S. Patent Nos. 5,395,760; 5,605,690, Re. 36,755.
Medication Guide
ENBREL® (en-brel)
(etanercept)
Read the Medication Guide that comes with ENBREL® before you start using it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. It is important to remain under your doctor’s care while using ENBREL®.
ENBREL® is a prescription medicine called a Tumor Necrosis Factor (TNF) blocker that affects your immune system.
What is the most important information I should know about ENBREL®?
ENBREL may cause serious side effects, including:
1. Risk of infection
ENBREL® can lower the ability of your immune system to fight infections. Some people have serious infections while taking ENBREL®. These infections include tuberculosis (TB), and infections caused by viruses, fungi or bacteria that spread throughout their body. Some people have died from these infections.
- Your doctor should test you for TB before starting ENBREL®.
- Your doctor should monitor you closely for symptoms of TB during treatment with ENBREL® even if you tested negative for TB.
- Your doctor should check you for symptoms of any type of infection before, during, and after your treatment with ENBREL®.
You should not start taking ENBREL® if you have any kind of infection unless your doctor says it is okay.
2. Risk of cancer
- There have been cases of unusual cancers in children and teenage patients who started using TNF-blocking agents at less than 18 years of age.
- For children, teenagers, and adults taking TNF-blocker medicines, including ENBREL, the chances of getting lymphoma or other cancers may increase.
- People with rheumatoid arthritis or psoriasis, especially those with very active disease, may be more likely to get lymphoma.
Before starting ENBREL®, be sure to talk to your doctor:
ENBREL® may not be right for you. Before starting ENBREL®, tell your doctor about all of your medical conditions, including:
Infections – tell your doctor if you:
- have an infection. (See “What is the most important information I should know about ENBREL®?”)
- are being treated for an infection
- think you have an infection
- have symptoms of an infection such as fever, sweats or chills, cough or flu-like symptoms, shortness of breath, blood in your phlegm, weight loss, muscle aches, warm, red, or painful areas on your skin, sores on your body, diarrhea or stomach pain, burning when you urinate or urinate more often than normal, and feel very tired.
- have any open cuts on your body
- get a lot of infections or have infections that keep coming back
- have diabetes, HIV, or a weak immune system. People with these conditions have a higher chance for infections.
- have TB, or have been in close contact with someone with TB
- were born in, lived in, or traveled to countries where there is a risk for getting TB. Ask your doctor if you are not sure.
- live, have lived in, or traveled to certain parts of the country (such as the Ohio and Mississippi River valleys, or the Southwest) where there is a greater risk for getting certain kinds of fungal infections (histoplasmosis, coccidioidomycosis, blastomycosis). These infections may happen or become more severe if you use ENBREL®. Ask your doctor if you do not know if you live or have lived in an area where these infections are common.
- have or have had hepatitis B
Also, BEFORE starting ENBREL®, tell your doctor:
-
About all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements including:
- Orencia® (abatacept) or Kineret® (anakinra). You have a higher chance for serious infections when taking ENBREL® with Orencia® or Kineret®.
- Cyclophosphamide (Cytoxan®). You may have a higher chance for getting certain cancers when taking ENBREL® with cyclophosphamide.
Keep a list of all your medications with you to show your doctor and pharmacist each time you get a new medicine. Ask your doctor if you are not sure if your medicine is one listed above.
Other important medical information you should tell your doctor BEFORE starting ENBREL®, including if you:
- have or had a nervous system problem such as multiple sclerosis
- have or had heart failure
- are scheduled to have surgery
- have recently received or are scheduled to receive a vaccine
- all vaccines should be brought up-to-date before starting ENBREL®
- people taking ENBREL® should not receive live vaccines
- ask your doctor if you are not sure if you received a live vaccine
- are allergic to rubber or latex
- the needle covers on the single-use prefilled syringes and the single-use prefilled SureClick® autoinjectors contains dry natural rubber
- have been around someone with varicella zoster (chicken pox)
- are pregnant or plan to become pregnant. It is not known if ENBREL® will harm your unborn baby.
- Pregnancy Registry: Amgen has a registry for pregnant women who take ENBREL®. The purpose of this registry is to check the health of the pregnant mother and her child. Talk to your doctor if you are pregnant and contact the registry at 1-877-311-8972.
- are breast feeding or plan to breast feed. It is not known if ENBREL® passes into your breast milk. You and your doctor should decide if you will take ENBREL® or breast feed. You should not do both.
See the section “What are the possible side effects of ENBREL®?” below for more information.
What is ENBREL®?
ENBREL® is a prescription medicine called a Tumor Necrosis Factor (TNF) blocker.
ENBREL® is used to treat:
- moderately to severely active rheumatoid arthritis (RA). ENBREL® can be used alone or with a medicine called methotrexate.
- psoriatic arthritis. ENBREL® can be used alone or with methotrexate.
- ankylosing spondylitis (AS)
- chronic, moderate to severe plaque psoriasis in adults ages 18 years and older
- moderately to severely active polyarticular juvenile idiopathic arthritis (JIA) in children ages 2 years and older.
You may continue to use other medicines that help treat your condition while taking ENBREL®, such as non-steroidal anti-inflammatory drugs (NSAIDs) and prescription steroids, as recommended by your doctor.
ENBREL® can help reduce joint damage, and the signs and symptoms of the above mentioned diseases. People with these diseases have too much of a protein called tumor necrosis factor (TNF), which is made by your immune system. ENBREL® can reduce the effect of TNF in the body and block the damage that too much TNF can cause, but it can also lower the ability of your immune system to fight infections. See “What is the most important information I should know about ENBREL® ?” and “What are the possible side effects of ENBREL® ?”
Who should not use ENBREL®?
Do not use ENBREL® if you:
- have an infection that has spread through your body (sepsis)
- have ever had an allergic reaction to ENBREL®. See the end of this Medication Guide for a complete list of ingredients in ENBREL®.
How should I use ENBREL®?
- ENBREL® is given as an injection under the skin (subcutaneous or SC).
- If your doctor decides that you or a caregiver can give the injections of ENBREL® at home, you or your caregiver should receive training on the right way to prepare and inject ENBREL®. Do not try to inject ENBREL® until you have been shown the right way by your doctor or nurse.
- ENBREL® is available in the forms listed below. Your doctor will prescribe the type that is best for you.
- Single-use Prefilled Syringe
- Single-use Prefilled SureClick Autoinjector
- Multiple-use Vial
- See the detailed “Patient Instructions for Use” with this Medication Guide for instructions about the right way to prepare and give your ENBREL® injections at home.
- Your doctor will tell you how often you should use ENBREL®. Do not miss any doses of ENBREL®. If you forget to use ENBREL®, inject your dose as soon as you remember. Then, take your next dose at your regular(ly) scheduled time. In case you are not sure when to inject ENBREL®, call your doctor or pharmacist. Do not use ENBREL® more often than as directed by your doctor.
- Your child’s dose of ENBREL® depends on his or her weight. Your child’s doctor will tell you which form of ENBREL® to use and how much to give your child.
What are the possible side effects of ENBREL®?
ENBREL® can cause serious side effects, including:
See “What is the most important information I should know about ENBREL® ?”
- Infections. ENBREL® can make you more likely to get infections or make any infection that you have worse. Call your doctor right away if you have any symptoms of an infection. See “Before starting ENBREL®, be sure to talk to your doctor” for a list of symptoms of infection.
- Hepatitis B infection in people who carry the virus in their blood. If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus can become active while you use ENBREL®. Your doctor may do a blood test before you start treatment with ENBREL® and while you use ENBREL®.
- Nervous system problems. Rarely, people who use TNF blocker medicines have developed nervous system problems such as multiple sclerosis, seizures, or inflammation of the nerves of the eyes. Tell your doctor right away if you get any of these symptoms: numbness or tingling in any part of your body, vision changes, weakness in your arms and legs, and dizziness.
- Blood problems. Low blood counts have been seen with other TNF blocker medicines. Your body may not make enough of the blood cells that help fight infections or help stop bleeding. Symptoms include fever, bruising or bleeding very easily, or looking pale.
- Heart failure including new heart failure or worsening of heart failure you already have. New or worse heart failure can happen in people who use TNF blocker medicines, like ENBREL®. If you have heart failure your condition should be watched closely while you take ENBREL®. Call your doctor right away if you get new or worsening symptoms of heart failure while taking ENBREL® such as shortness of breath or swelling of your lower legs or feet.
- Psoriasis. Some people using ENBREL® developed new psoriasis or worsening of psoriasis they already had. Tell your doctor if you develop red scaly patches or raised bumps which may be filled with pus . Your doctor may decide to stop your treatment with ENBREL®.
- Allergic reactions. Allergic reactions can happen to people who use TNF blocker medicines. Call your doctor right away if you have any symptoms of an allergic reaction. Symptoms of an allergic reaction include a severe rash, a swollen face, or trouble breathing.
-
Autoimmune reactions, including:
- Lupus-like syndrome. Symptoms include a rash on your face and arms that gets worse in the sun. Tell your doctor if you have this symptom. Symptoms may go away when you stop using ENBREL®.
- Autoimmune hepatitis. Liver problems can happen in people who use TNF blocker medicines, including ENBREL®. These problems can lead to liver failure and death. Call your doctor right away if you have any of these symptoms: feel very tired, skin or eyes look yellow, poor appetite or vomiting, pain on the right side of your stomach (abdomen).
Common side effects of ENBREL® include:
- Injection site reactions such as redness, swelling, itching, or pain. These symptoms usually go away within 3 to 5 days. If you have pain, redness or swelling around the injection site that doesn’t go away or gets worse, call your doctor.
- Upper respiratory infections (sinus infections)
- Headache
These are not all the side effects with ENBREL®. Tell your doctor about any side effect that bothers you or does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ENBREL®?
- Store ENBREL® in the refrigerator at 36° to 46°F (2° to 8°C).
- Do not freeze.
- Do not shake.
- Keep ENBREL® in the original carton to protect from light.
- Keep ENBREL® and all medicines out of the reach of children.
General Information about ENBREL®
Medicines are sometimes prescribed for purposes not mentioned in a Medication Guide. Do not use ENBREL® for a condition for which it was not prescribed. Do not give ENBREL® to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about ENBREL®. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ENBREL® that was written for healthcare professionals. For more information call 1-888-4ENBREL (1-888-436-2735).
What are the ingredients in ENBREL®?
Single-use Prefilled Syringe and the Single-use Prefilled SureClick Autoinjector:
Active Ingredient: etanercept
Inactive Ingredients: sucrose, sodium chloride, L-arginine hydrochloride and sodium phosphate
Multiple-use Vial:
Active Ingredient: etanercept
Inactive Ingredients: mannitol, sucrose, tromethamine.
[Amgen Logo]
[Wyeth Logo]
Manufactured by:
Immunex Corporation
Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc. and Wyeth Pharmaceuticals
© 1998 – 2009 Immunex Corporation. All rights reserved.
1XXXXXX – v2.5
Issue Date: xx/xxxx
Printed in the U.S.A.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Patient Instructions for Use
ENBREL® (en-brel)
(etanercept)
Single-use Prefilled Syringe
How do I prepare and give an injection with ENBREL® Single-use Prefilled Syringe?
There are 2 types of ENBREL® single-use prefilled syringes:
- The 50 mg single-use prefilled syringe that contains one 50 mg dose of ENBREL®.
- The 25 mg single-use prefilled syringe that contains one 25 mg dose of ENBREL®.
Your doctor will tell you which one to use.
A 50 mg dose can be given as one injection using a 50 mg single-use prefilled syringe or as two injections using 25 mg single-use prefilled syringes. Your doctor will tell you whether the two injections with 25 mg single-use prefilled syringes should be given on the same day once a week or on two different days (3 or 4 days apart) in the same week.
Children must weigh at least 138 pounds to use the ENBREL® 50 mg single-use prefilled syringe. Children who weigh less than 138 pounds should use a different form of ENBREL®. The ENBREL® 25 mg single-use prefilled syringe should not be used in pediatric patients weighing less than 68 pounds.
IMPORTANT: The needle cover on the single-use prefilled syringe contains latex. Tell your doctor if you are allergic to latex.
STEP 1: Setting Up for an Injection
- Select a clean, well-lit, flat work surface, such as a table.
- Take the ENBREL® carton containing the prefilled syringes out of the refrigerator and place it on your flat work surface. Remove one prefilled syringe and place it on your work surface. Do not shake the prefilled syringe of ENBREL®. Place the carton containing any remaining prefilled syringes back into the refrigerator (2° to 8°C [36° to 46°F]). If you have any questions about storage, contact your doctor, nurse, or pharmacist for further instructions.
- Check the expiration date on the prefilled syringe. If the expiration date has passed, do not use the prefilled syringe and contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- Wait 15 to 30 minutes to allow the ENBREL® in the prefilled syringe to reach room temperature. DO NOT remove the needle cover while allowing it to reach room temperature. Do not warm ENBREL® in any other way (for example, do not warm it in a microwave or in hot water).
- Hold the prefilled syringe with the covered needle pointing down. If bubbles are seen in the syringe, very gently tap the prefilled syringe to allow any bubbles to rise to the top of the syringe. Turn the syringe so that the purple horizontal lines on the barrel are directly facing you. Check to see if the amount of liquid in the syringe falls between the purple lines. The top of the liquid may be curved. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE. Contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- Assemble the additional supplies you will need for your injection. These include an alcohol swab, a cotton ball or gauze, and a puncture-resistant disposal container.
- Wash your hands with soap and warm water.
- Make sure the solution in the prefilled syringe is clear and colorless. You may notice small white particles in the solution. These particles are formed from ENBREL® and this is acceptable. However, do not inject the solution if it is cloudy or discolored, or contains large or colored particles – contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
STEP 2: Choosing and Preparing an Injection Site
- Three recommended injection sites for ENBREL® using a prefilled syringe include: (1) the front of the middle thighs; (2) the abdomen, except for the two-inch area right around the navel; and, (3) the outer area of the upper arms.
- Rotate the site for each injection. Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
- If you have psoriasis, you should try not to inject directly into any raised, thick, red, or scaly skin patches (“psoriasis skin lesions”).
- To prepare the area of skin where ENBREL® is to be injected, wipe the injection site with an alcohol swab. Do not touch this area again before giving the injection.
STEP 3: Injecting ENBREL® Using a Prefilled Syringe
- Pick up the prefilled syringe from your flat work surface. Hold the barrel of the prefilled syringe with one hand and pull the needle cover straight off.
When you remove the needle cover, there may be a drop of liquid at the end of the needle; this is normal. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out. - Holding the syringe with the needle pointing up, check the syringe for air bubbles. If there are bubbles, gently tap the syringe with your finger until the air bubbles rise to the top of the syringe. Slowly push the plunger up to force the air bubbles out of the syringe.
- Holding the syringe in one hand like a pencil, use the other hand to gently pinch a fold of skin at the cleaned injection site and hold it firmly.
- Insert the needle at a slight angle (45 degrees) to the skin. With a quick, “dart-like” motion, insert the needle into the skin.
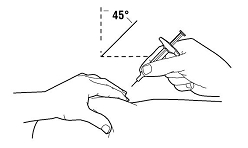
- After the needle is inserted, let go of the skin. Pull the plunger back slightly. If blood comes into the syringe, do not inject ENBREL® because the needle has entered a blood vessel. Withdraw the needle and discard it in a puncture-resistant container. Repeat the steps to prepare for an injection using a new prefilled syringe of ENBREL®. Do not use the same prefilled syringe.
- If no blood appears in the syringe, slowly push the plunger all the way down to inject ENBREL®.
- When the syringe is empty, pull the needle out of the skin, being careful to keep it at the same angle as inserted. There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site for 10 seconds. Do not rub the injection site. If needed, you may cover the injection site with a bandage.
STEP 4: Disposing of Supplies
The syringe should NEVER be reused. NEVER recap a needle.
Dispose of the used syringe as instructed by your healthcare provider, or by following these steps:
- Do not throw the used syringe in the household trash or recycle.
- Place the used syringe in a hard plastic disposal container with a screw-on cap or a metal container with a plastic lid, such as a coffee can, labeled “used syringes.” If a metal container is used, cut a small hole in the plastic lid and tape the lid to the metal container. If a hard plastic container is used, always screw the cap on tightly after each use. Do not use glass or clear plastic containers. Puncture-resistant containers may also be purchased at your local pharmacy.
- When the container is full, tape around the cap or lid to make sure the cap or lid does not come off.
- You should always check first with your healthcare provider for instructions on how to properly dispose of a filled disposal container. There may be special state and local laws for disposing of used needles and syringes. Do not throw the disposal container in household trash. Do not recycle.
- Always keep the container out of the reach of children.
A healthcare provider familiar with ENBREL® should answer all questions. A toll-free information service is also available: 1-888-4ENBREL (1-888-436-2735).
[Amgen]
[Wyeth]
Manufactured by:
Immunex Corporation,
Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc. and Wyeth Pharmaceuticals
© 1998 – 2008 Immunex Corporation. All rights reserved.
1XXXXXX - v1
Issue Date: 06/2008
Patient Instructions for Use
ENBREL® (en-brel)
(etanercept)
Multiple-use Vial
How do I prepare and give an injection with ENBREL® Multiple-use Vial?
A multiple-use vial contains 25 mg of ENBREL®.
STEP 1: Setting up for an Injection
- Select a clean, well-lit, flat work surface, such as a table.
- Take the ENBREL® dose tray out of the refrigerator and place it on your flat work surface.
- Check the expiration date on the dose tray. If the expiration date has passed, do not use the dose tray. Also check to make sure the dose tray has seven items as pictured below:
-
- One prefilled diluent syringe containing 1 mL of diluent (liquid) with attached adapter and twist-off cap
- One plunger
- One ENBREL® vial
- One 27-gauge ½ inch needle in hard plastic cover
- One vial adapter
- Two alcohol swabs
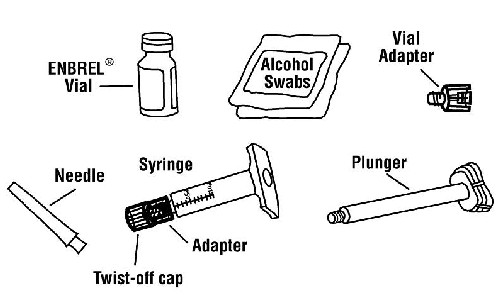
If the expiration date has passed, the seven items are not included in the dose tray or if any item looks damaged, contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- Wash your hands with soap and warm water.
- Peel the paper seal off the dose tray and remove all items.
- Inspect the volume of diluent in the syringe with the twist-off cap pointing down. Use the unit markings on the side of the syringe to make sure there is at least 1 mL of liquid in the syringe. If the level of liquid is below the 1 mL mark, do not use. Call 1-888-4ENBREL (1-888-436-2735) for assistance.
STEP 2: Preparing the ENBREL® Solution
There are two methods for preparing the ENBREL® solution. For some children, one vial of ENBREL® solution can be used for more than one dose. The free-hand method should be used for children on ENBREL® who are using one vial of ENBREL® solution for more than one dose. You should not use the vial adapter method if you will be using the vial more than once. Ask your healthcare provider if you have questions about which method to use.
- The Vial Adapter Method
Adult patients and larger children on ENBREL® may use the vial adapter device to assist with mixing the powder with the liquid and withdrawing ENBREL®, and then use a 27-gauge needle to inject the dose. This method should not be used for children using multiple doses from the same vial of ENBREL®. The instructions for using the vial adapter method are in STEP 2A.
- The Free-Hand Method
In the free-hand method, a 25-gauge needle is used to assist with mixing the powder with the liquid and withdrawing ENBREL®, and a 27-gauge needle is used to inject the dose. Obtain 25-gauge needles from your healthcare provider. Instructions for using the free-hand method are in STEP 2B.
The instructions for preparing additional doses from the same vial of ENBREL® solution are in STEP 3. For each additional dose, you will need two new needles (one 25-gauge needle to withdraw the solution and one 27-gauge needle for injection) and one new empty syringe (1 mL). NEVER REUSE A SYRINGE OR NEEDLE.
If you are using the vial of ENBREL® for more than one dose, you should write the date you mixed the powder and liquid in the area marked “Mixing Date:” on the sticker supplied with these instructions, and attach the sticker to the ENBREL® vial.
After you have withdrawn the dose of ENBREL® that you need, store the ENBREL® vial (in the dose tray) in the refrigerator at 36° to 46°F (2° to 8°C) as soon as possible, but always within 4 hours of mixing the solution.
The ENBREL® solution must be used within 14 days of the mixing date. You should discard the ENBREL® vial and any remaining solution if it is not used within 14 days. Do not mix any remaining liquid in one vial of ENBREL® solution with another.
STEP 2A: Vial Adapter Method
- Remove the pink plastic cap from the ENBREL® vial. Do not remove the gray stopper or silver metal ring around the top of the ENBREL® vial.
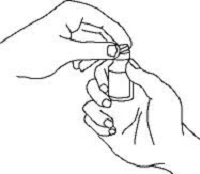
- Place the ENBREL® vial on your flat work surface or turn your dose tray upside down and place your ENBREL® vial in the round space marked “V”. Use one alcohol swab to clean the gray stopper on the ENBREL® vial. Do not touch the gray stopper with your hands.
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use.
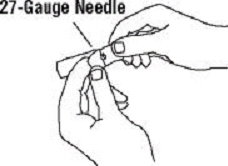
- Open the wrapper that contains the vial adapter by peeling apart the tabs and set the vial adapter aside for later use. Do not touch the vial adapter’s twist-on end or the spike inside.
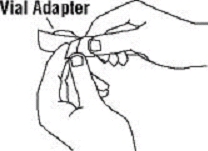
- Slide the plunger into the flange end of the syringe.
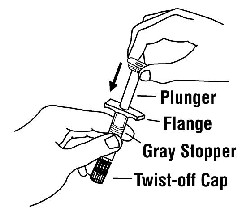
- Attach the plunger to the gray rubber stopper in the syringe by turning the plunger clockwise until a slight resistance is felt.
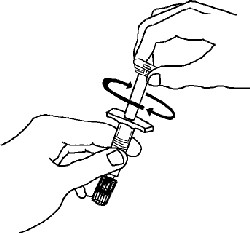
- Remove the twist-off cap from the prefilled diluent syringe by turning counter-clockwise. Do not bump or touch the plunger. Doing so could cause the liquid to leak out. You may see a drop of liquid when removing the cap. This is normal. Place the cap on your flat work surface. Do not touch the syringe tip.
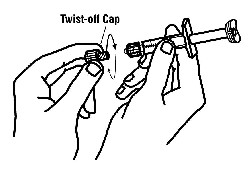
- Once the twist-off cap is removed, pick up the vial adapter with your free hand. Twist the vial adapter onto the syringe until a slight resistance is felt. Do not over-tighten.
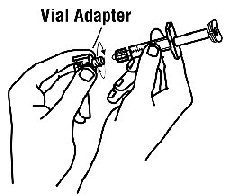
- Hold the ENBREL® vial upright on your flat work surface. Grasp the sides of the vial adapter and place it over the top of the ENBREL® vial. Do not bump or touch the plunger. Doing so could cause the liquid to leak out. Insert the vial adapter into the gray stopper on the ENBREL® vial. The plastic spike inside the vial adapter should puncture the gray stopper. The vial adapter should fit snugly.
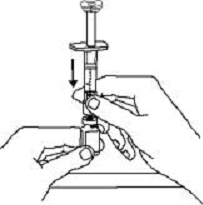
- Hold the ENBREL® vial upright on your flat work surface and push the plunger down until all the liquid from the syringe is in the ENBREL® vial. You may see foaming (bubbles) in the vial. This is normal.
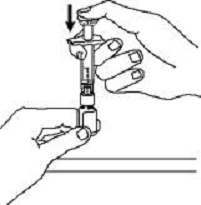
- Gently swirl the ENBREL® vial in a circular motion to dissolve the powder. If you used the dose tray to hold your ENBREL® vial, take the vial (with the vial adapter and syringe still attached) out of the dose tray, and gently swirl the vial in a circular motion to dissolve the powder.
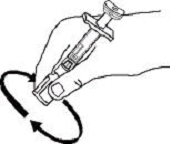
DO NOT SHAKE. Wait until all the powder dissolves (usually less than 10 minutes). The solution should be clear and colorless. After the powder has completely dissolved, foam (bubbles) may still be present. This is normal. Do not inject the solution if it is discolored, contains lumps, flakes, or particles. If all the powder in the ENBREL® vial is not dissolved or there are particles present after 10 minutes, call 1-888-4ENBREL (1-888-436-2735) for assistance. - Turn the ENBREL® vial upside down. Hold the syringe at eye level and slowly pull the plunger down to the unit markings on the side of the syringe that correspond with your/your child’s dose. For adult patients, remove the entire volume (1 mL), unless otherwise instructed by your doctor. Be careful not to pull the plunger completely out of the syringe. Some white foam may remain in the ENBREL® vial. This is normal.
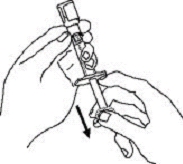
- Check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger to again draw the correct amount of solution back into the syringe.
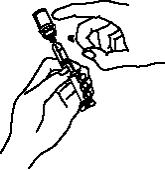
- Remove the syringe from the vial adapter, by holding the vial adapter with one hand and turning the syringe counterclockwise with your other hand. Do not touch or bump the plunger. Place the ENBREL® vial with the vial adapter on your flat work surface.
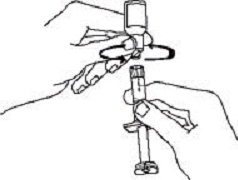
- Continue to hold the barrel of the syringe. With your free hand, twist the 27-gauge needle onto the tip of the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject ENBREL®.
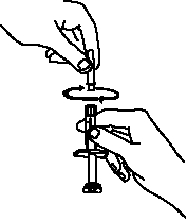
GO TO STEP 4: CHOOSING AND PREPARING AN INJECTION SITE.
STEP 2B: Free-Hand Method
If you are preparing a dose from an ENBREL® vial that was previously used, go to STEP 3: Preparing Additional Doses from a Single ENBREL® Vial.
- Remove the pink plastic cap from the ENBREL® vial. Do not remove the gray stopper or silver metal ring around the top of the ENBREL® vial. Write the date you mix the powder and solution on the supplied “Mixing Date:” sticker and attach it to the ENBREL® vial.
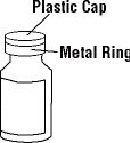
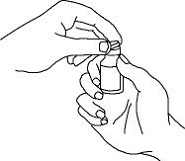
- Place the ENBREL® vial on your flat work surface. Use one alcohol swab to clean the gray stopper on the ENBREL® vial. Do not touch the gray stopper with your hands.
- Open the wrapper that contains the 25-gauge needle by peeling apart the tabs and set the needle aside for later use. The 25-gauge needle will be used to mix the liquid with the powder and for withdrawing ENBREL® from the vial.
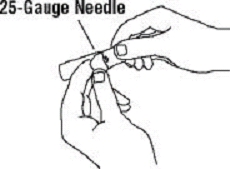
- Slide the plunger into the flange end of the syringe.
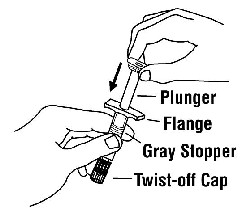
- Attach the plunger to the gray rubber stopper in the syringe by turning the plunger clockwise until a slight resistance is felt.
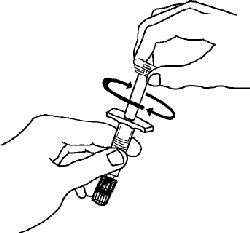
- Remove the twist-off cap from the prefilled diluent syringe by turning counter-clockwise. Do not touch or bump the plunger. Doing so could cause the liquid to leak out. You may see a drop of liquid when removing the cap. This is normal. Place the cap on your flat work surface. Do not touch the syringe tip.
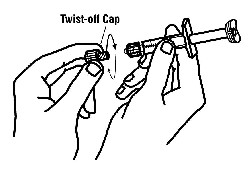
- Continue to hold the barrel of the syringe. With your free hand, twist the 25-gauge needle onto the tip of the syringe until it fits snugly. Place the syringe on your flat work surface.
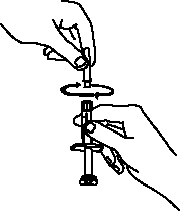
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use. The 27-gauge needle will be used to inject the dose.
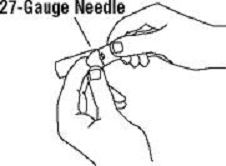
- Pick up the syringe from your flat work surface. Hold the barrel of the syringe with one hand, and pull the needle cover straight off. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out.
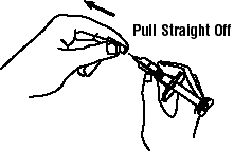
- Place the needle cover (open side up) in the round space marked “N” in the ENBREL® dose tray.
- Place the ENBREL® vial on your flat work surface. Hold the syringe with the needle facing up, and gently pull back on the plunger to pull a small amount of air into the syringe. Then, insert the needle straight down through the center ring of the gray stopper (see illustrations). You should feel a slight resistance and then a “pop” as the needle goes through the center of the stopper. Look for the needle tip inside the open stopper window. If the needle is not correctly lined up with the center of the stopper, you will feel constant resistance as it goes through the stopper and no “pop”. The needle may enter at an angle and bend, break or prevent you from adding diluent into the ENBREL® vial.
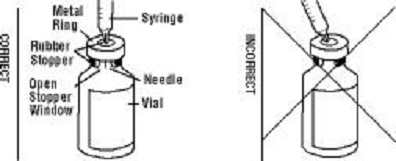
- Push the plunger down VERY SLOWLY until all liquid from the syringe is in the ENBREL® vial. Adding the liquid too fast will cause foaming (bubbles).
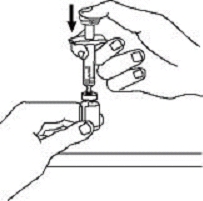
- Leave the syringe in place. Gently swirl the ENBREL® vial in a circular motion to dissolve the powder.
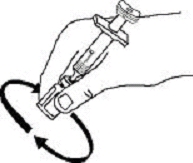
DO NOT SHAKE. Wait until all the powder dissolves (usually less than 10 minutes). The solution should be clear and colorless. After the powder has completely dissolved, foam (bubbles) may still be present. This is normal. Do not inject the solution if it is discolored, contains lumps, flakes, or particles. If all the powder in the ENBREL® vial is not dissolved or there are particles present after 10 minutes, call 1-888-4ENBREL (1-888-436-2735) for assistance. - With the needle in the ENBREL® vial, turn the vial upside down. Hold the syringe at eye level and slowly pull the plunger down to the unit markings on the side of the syringe that correspond with the correct dose. Make sure to keep the tip of the needle in the solution. Some white foam may remain in the ENBREL® vial. This is normal.
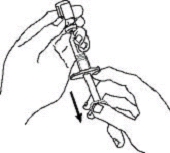
- With the needle still inserted in the ENBREL® vial, check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the vial, slowly pull back on the plunger to draw the correct amount of solution back into the syringe.
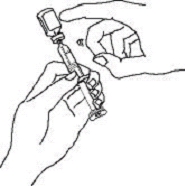
- Remove the syringe and needle from the ENBREL® vial. Keep the needle attached to the syringe and insert the 25-gauge needle straight down into the needle cover in the ENBREL® dose tray.
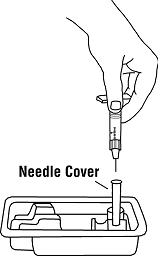
You should hear a “snap” when the needle is secure in the needle cover. Once the needle is secure in the needle cover, untwist the 25-gauge needle from the syringe and dispose of the needle in your SHARPS container. - Twist the 27-gauge needle onto the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject ENBREL®.
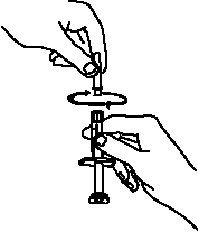
- If there is enough solution left in the ENBREL® vial for another dose, write the date you mixed the powder and liquid in the area marked “Mixing Date:” on the sticker supplied with these instructions, and attach the sticker to the ENBREL® vial. Refrigerate the ENBREL® vial (in the dose tray) after use. Prepare additional doses from the ENBREL® vial as described in STEP 3. Otherwise, discard the ENBREL® vial and any remaining solution.
GO TO STEP 4: CHOOSING AND PREPARING AN INJECTION SITE.
STEP 3: Preparing Additional Doses from a Single ENBREL® Vial
- Select a clean, well-lit, flat work surface, such as a table.
- The needles and syringes supplied with ENBREL® should not be reused. You will need new ones for each additional dose. Your healthcare provider will tell you what type of syringes (1 mL) and needles (25- and 27-gauge) to use. Alcohol swabs are available at the drug store. Place the sterile syringe with a 25-gauge needle (for withdrawing ENBREL®), a 27-gauge needle (for injecting ENBREL®) and two alcohol swabs on your flat work surface.
- Take the vial of ENBREL® solution that is stored in the dose tray out of the refrigerator and place it on your flat work surface.
- Check the mixing date you wrote on the sticker on the ENBREL® vial. Discard the ENBREL® vial if more than 14 days have passed since the ENBREL® solution was mixed.
- Wash your hands with soap and warm water.
- Use one alcohol swab to clean the gray stopper on the ENBREL® vial. Do not touch the stopper with your hands.
- If the syringe and the 25-gauge needle are not pre-assembled, assemble them as instructed by your healthcare provider.
- Open the wrapper that contains the 27-gauge needle by peeling apart the tabs and set the needle aside for later use. The 27-gauge needle will be used to inject the dose of ENBREL®.
- Hold the syringe and pull the needle cover straight off. Do not touch the needle or allow it to touch any surface. Place the needle cover (open side up) in the round space marked “N” in the ENBREL® dose tray.
- Place the ENBREL® vial on your flat work surface. Hold the syringe with the needle facing up, and gently pull back the plunger to pull a small amount of air into the syringe. Then, insert the 25-gauge needle straight down through the center ring of the gray stopper. You should feel a slight resistance and then a “pop” as the needle goes through the center of the stopper. Look for the needle tip inside the open stopper window. If the needle is not correctly lined up with the center of the stopper, you will feel constant resistance as it goes through the stopper and no “pop”. The needle may enter at an angle and bend, break, or prevent proper withdrawal of ENBREL® solution from the vial.
- Keep the needle in the ENBREL® vial and turn the vial upside down. Hold the syringe at eye level, and slowly pull the plunger down to the unit markings on the syringe that correspond to your child’s dose. As the amount of solution in the ENBREL® vial drops, you may need to pull the needle back just enough to keep the tip of the needle in the solution.
- With the needle still inserted in the ENBREL® vial, check for air bubbles in the syringe. Gently tap the syringe to make any air bubbles rise to the top of the syringe. Slowly push the plunger up to remove the air bubbles. If you push solution back into the ENBREL® vial, slowly pull back on the plunger to again draw the correct amount of solution back into the syringe.
- Remove the syringe and needle from the ENBREL® vial. Keep the needle attached to the syringe and insert the 25-gauge needle straight down into the needle cover in the ENBREL® dose tray. You should hear a “snap” when the needle is secure in the needle cover. Once the needle is secure in the needle cover, remove the 25-gauge needle from the syringe and dispose of the needle in a puncture-resistant container.
- Attach the 27-gauge needle onto the tip of the syringe until it fits snugly. Do not remove the needle cover from the syringe. Place the syringe on your flat work surface until you are ready to inject ENBREL®.
STEP 4: Choosing and Preparing an Injection Site
- Three recommended injection sites for ENBREL® include: (1) the front of the middle thighs; (2) the abdomen, except for the two-inch area right around the navel; and, (3) the outer area of the upper arms.
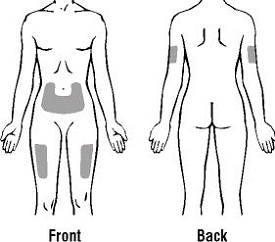
- Rotate the site for each injection. Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
- If you have psoriasis, you should try not to inject directly into any raised, thick, red, or scaly skin patches or lesions.
- To prepare the area of skin where ENBREL® is to be injected, wipe the injection site with a new alcohol swab. Do not touch this area again before giving the injection.
STEP 5: Injecting the ENBREL® Solution
- Pick up the syringe from your flat work surface. Hold the barrel of the syringe with one hand and pull the needle cover straight off. Do not touch the needle or allow it to touch any surface. Do not touch or bump the plunger. Doing so could cause the liquid to leak out.
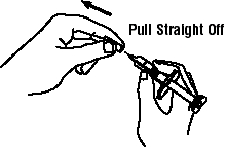
- With one hand, gently pinch the cleaned area of skin and hold it firmly. With the other hand, hold the syringe (like a pencil) at a 45-degree angle to the skin.
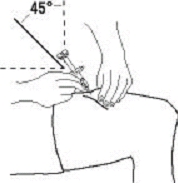
- With a quick, "dart-like" motion, push the needle into the skin.
- After the needle is inserted, let go of the skin. Pull the plunger back slightly. If no blood appears in the syringe, slowly push the plunger all the way down to inject ENBREL®.
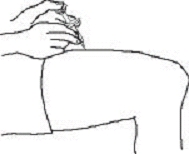
If blood comes into the syringe, do not inject ENBREL® because the needle has entered a blood vessel. Withdraw the needle and repeat the steps to prepare for an injection. Do not use the same syringe and needle. Dispose of the used needle and syringe in a puncture-resistant container. - When the syringe is empty, pull the needle out of the skin, being careful to keep it at the same angle as inserted.
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site for 10 seconds. Do not rub the injection site. If needed, you may cover the injection site with a bandage.
- If your doctor has instructed you to take two ENBREL® injections on the same day, repeat the steps to prepare and give an injection of ENBREL®. Choose and prepare a new injection site for the second injection.
- FOR FREE-HAND METHOD - If there is enough solution left in the ENBREL® vial for another dose, refrigerate the ENBREL® vial (in the dose tray) after use. Otherwise, discard the ENBREL® vial and any remaining solution.
STEP 6: Disposing of Supplies
The syringe, needles, and vial adapter should NEVER be reused. NEVER recap a needle.
Dispose of both the used needle and syringe as instructed by your healthcare provider, or by following these steps:
- Do not throw the used needle or syringe in the household trash or recycle.
- Place the used needle and syringe in a hard plastic disposal container with a screw-on cap or a metal container with a plastic lid, such as a coffee can, labeled “used syringes.” If a metal container is used, cut a small hole in the plastic lid and tape the lid to the metal container. If a hard plastic container is used, always screw the cap on tightly after each use. Do not use glass or clear plastic containers. Puncture-resistant containers may also be purchased at your local pharmacy.
- When the container is full, tape around the cap or lid to make sure the cap or lid does not come off.
- You should always check first with your healthcare provider for instructions on how to properly dispose of a filled disposal container. There may be special state and local laws for disposing of used needles and syringes. Do not throw the disposal container in household trash. Do not recycle.
- Always keep the container out of the reach of children.
- The ENBREL® vials, vial adapters, and used alcohol swabs should be placed in the trash. The dose tray and cover may be recycled.
A healthcare provider familiar with ENBREL® should answer all questions. A toll-free information service is also available: 1-888-4ENBREL (1-888-436-2735).
[Amgen]
[Wyeth]
Manufactured by:
Immunex Corporation,
Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc. and Wyeth Pharmaceuticals
© 1998 – 2008 Immunex Corporation. All rights reserved.
1XXXXXX - v1
Issue Date: 06/2008
Patient Instructions for Use
ENBREL® (en-brel)
(etanercept)
Single-use Prefilled SureClick™ Autoinjector
How do I prepare and give an injection with ENBREL® Single-use Prefilled SureClick™ Autoinjector?
This section contains information on how to properly use the ENBREL® SureClick™ autoinjector. It is important that you do not try to give yourself the injection unless you have received special training from your doctor, nurse or pharmacist.
A single-use prefilled SureClick™ autoinjector contains one 50 mg dose of ENBREL®.
Children must weigh at least 138 pounds to use ENBREL® single-use prefilled Sureclick™ autoinjector. Children who weigh less than 138 pounds should use a different form of ENBREL®.
IMPORTANT: The needle cap on the single-use prefilled SureClick™ autoinjector contains latex. Tell your doctor if you are allergic to latex.
Equipment:
- A new ENBREL® SureClick™ autoinjector, and
- Alcohol wipes or similar
- Cotton ball or gauze
- Puncture resistant disposable container
Preparing for an ENBREL® Injection
- Find a comfortable, well-lit, clean surface and put all the equipment you need within reach.
- Remove one single-use prefilled ENBREL® SureClick™ autoinjector from the carton. Do not shake the autoinjector. Place the carton containing any remaining autoinjectors back into the refrigerator (2° to 8°C [36° to 46°F]). Do not freeze. If you have any questions about storage, contact your doctor, nurse, or pharmacist for further instructions.
- Check the expiration date on the autoinjector label. Do not use if the date has passed the last day of the month shown.
- Make sure the solution in the autoinjector is clear and colorless by looking through the inspection window. You may notice small white particles in solution. These particles are formed from ENBREL® and this is acceptable. However, do not inject the solution if it is cloudy or discolored, or contains large or colored particles - contact your pharmacist or call 1-888-4ENBREL (1-888-436-2735) for assistance.
- For a more comfortable injection, wait 15 to 30 minutes to allow the autoinjector to reach room temperature. Do not remove the white needle cap while allowing it to reach room temperature. Do not warm ENBREL® in any other way (for example, do not warm it in a microwave or in hot water).
- Wash your hands thoroughly.
- Do not remove the white needle cap from the autoinjector until you are ready to inject.

Choosing and Preparing an Injection Site
Choose an Injection Site
The injection site must be firm for the autoinjector to work properly. The preferred injection site for ENBREL® using a prefilled SureClick™ autoinjector is the front of the thigh.
-
Rotate Injection Location
Rotate the site for each injection. Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with stretch marks. -
Patients with Psoriasis
If you have psoriasis, you should try not to inject directly into any raised, thick, red, or scaly skin patches or lesions.
Instructions for Preferred Injection Site
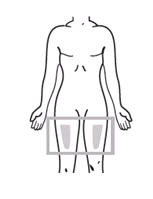 | The front of the thigh is the preferred injection site. | 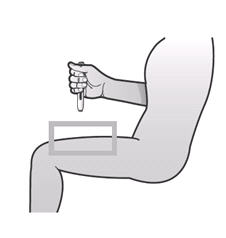 |
Instructions for Alternate Injection Sites
When using alternate injection sites, it is particularly important to create enough firmness on the site to be able to successfully complete the injection.
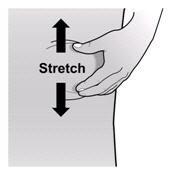 |
Stretch technique Make sure the skin under and around the prefilled autoinjector is firm and taut to provide enough resistance to fully retract the safety guard and unlock the prefilled autoinjector. |
 | You can use the abdomen, except for the two-inch area right around the navel. Stretch the skin at the injection site to create a firm and taut surface. |
 | If someone else is giving you the injection, they can also use the outer area of the upper arms. For the upper arms, it is recommended to stretch the skin at the injection site to create a firm and taut surface. |
Prepare the site
To prepare the area of skin where ENBREL® is to be injected, wipe the injection site with an alcohol swab. Do not touch this area again before giving the injection.
Injecting ENBREL® Using a Single-use Prefilled SureClick™ Autoinjector
1. Pull off the white needle cap.
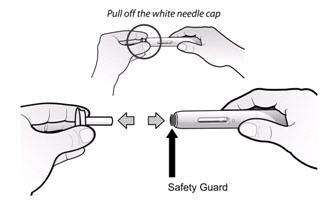
| ⃠ | Do not twist the white needle cap |
| ⃠ | Do not re-attach needle cap onto the prefilled autoinjector |
| ✔ | Do pull the white cap straight off. |
2. Do not touch the purple button. Press prefilled autoinjector onto skin to unlock safety guard.
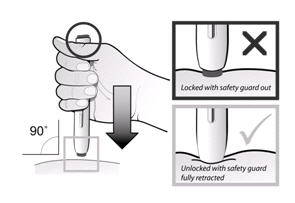
| ⃠ | Do not press the purple button until the safety guard is fully retracted. |
| ✔ | Do keep enough downward pressure to fully retract the purple safety guard and to keep the purple button unlocked. |
| ✔ | Do hold the prefilled autoinjector at a right angle (90º) at the injection site. |
3. Briefly press and release purple button.
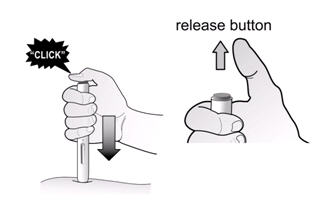
| ✔ | Do maintain pressure on the skin during the injection. |
4. Count slowly to 15 seconds for injection to end.
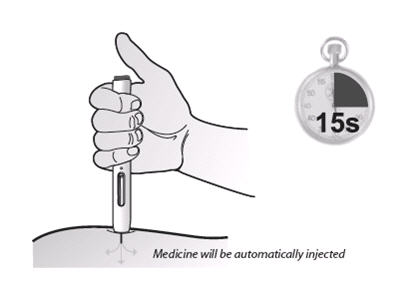
| ⃠ | Do not move the prefilled autoinjector during the injection. |
| ✔ | Do wait for the injection to finish before releasing pressure. |
| ✔ | You might hear a second click as the purple button pops back up. |
After the Injection
- Check window to confirm delivery of full dose.
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site.
- Do not rub the injection site. If needed, you may cover the injection site with a bandage.
Remember
If you have any problems, please call 1-888-4ENBREL (1-888-436-2735) for assistance.
Disposing of Supplies
The SureClick™ autoinjector should NEVER be reused.
Dispose of the used autoinjector as instructed by your healthcare provider, or by following these steps:
- Do not throw the used autoinjector in the household trash or recycle.
- Place the used autoinjector in a hard plastic disposal container with a screw-on cap or a metal container with a plastic lid, such as a coffee can, labeled “used syringes.” If a metal container is used, cut a small hole in the plastic lid and tape the lid to the metal container. If a hard plastic container is used, always screw the cap on tightly after each use. Do not use glass or clear plastic containers. Puncture-resistant containers may also be purchased at your local pharmacy.
- When the container is full, tape around the cap or lid to make sure the cap or lid does not come off.
- You should always check first with your healthcare provider for instructions on how to properly dispose of a filled disposal container. There may be special state and local laws for disposing of used needles and syringes, including autoinjectors. Do not throw the disposal container in household trash. Do not recycle.
- Always keep the container out of the reach of children.
A healthcare provider familiar with ENBREL® should answer all questions. A toll free information service is also available: 1 888 4ENBREL (1 888 436 2735).
[Amgen]
[Wyeth]
Manufactured by:
Immunex Corporation,
Thousand Oaks, CA 91320-1799
Marketed by Amgen Inc. and Wyeth Pharmaceuticals
© 1998 – 2008 Immunex Corporation. All rights reserved.
1XXXXXX - v1
Issue Date: 06/2008
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - PREFILLED SYRINGE, 25 MG
Contains 4 Single-use Prefilled Syringes
NDC 58406-455-04
Enbrel®
etanercept
25 mg/0.5 mL
Single-use Prefilled Syringe
25 mg
Attention: Not for use in pediatric patients under 68 lbs.
For Subcutaneous Use Only
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-use prefilled syringe contains 0.51 mL of a clear and colorless solution containing 50 mg/mL etanercept and is formulated at pH 6.3 ± 0.2, with 1% sucrose, 100 mM sodium chloride, 25 mM L-arginine hydrochloride, and 25 mM sodium phosphate.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
Marketed by Amgen and Wyeth Pharmaceuticals
©2004 Immunex Corporation
Immunex U.S. Patent Numbers: 5,395,760; 5,605,690; 5,945,397; 6,201,105; Re. 36,755

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - PREFILLED SYRINGE, 50 MG
Contains 4 Single-use Prefilled Syringes
NDC 58406-435-04
Enbrel®
etanercept
50 mg/mL
Single-use Prefilled Syringe
50 mg
Attention: Not for use in pediatric patients under 138 lbs.
For Subcutaneous Use Only
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-use prefilled syringe contains 0.98 mL of a clear and colorless solution containing 50 mg/mL etanercept and is formulated at pH 6.3 ± 0.2, with 1% sucrose, 100 mM sodium chloride, 25 mM L-arginine hydrochloride, and 25 mM sodium phosphate.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
Marketed by Amgen and Wyeth Pharmaceuticals
©2004 Immunex Corporation
Immunex U.S. Patent Numbers: 5,695,760; 5,605,690; 5,945,397; 6,201,105; Re. 36,755

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - PREFILLED AUTOINJECTOR, 50 MG
Contains 4 Single-use Prefilled Autoinjectors
NDC 58406-445-04
Enbrel®
etanercept
SureClick® Autoinjector
50 mg/mL
Single-use Prefilled Autoinjector
50 mg
Attention: Not for use in pediatric patients under 138 lbs.
For Subcutaneous Use Only
Sterile Solution – No Preservative
Refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
Each single-use prefilled autoinjector contains 0.98 mL of a clear and colorless solution containing 50 mg/mL etanercept and is formulated at pH 6.3 ± 0.2, with 1% sucrose, 100 mM sodium chloride, 25 mM L-arginine hydrochloride, and 25 mM sodium phosphate.
Specific activity: approximately 1.7 x 106 U/mg.
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
Marketed by Amgen and Wyeth Pharmaceuticals
©2004 Immunex Corporation
Immunex U.S. Patent Numbers: 5,395,760; 5,605,690; 5,945,397; 6,201,105; Re. 36,755

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - VIAL, 25 MG
Contains 4 Dose Trays
NDC 58406-425-34
Amgen®
Enbrel®
etanercept
25 mg/vial
Multiple-use Vial
See package insert for full prescribing information and instructions for preparation and administration.
25 mg/vial
Each vial contains a sterile lyophilized preparation of 25 mg etanercept (a recombinant CHO cell-derived product), 40 mg mannitol, 10 mg sucrose, and 1.2 mg tromethamine.
Specific activity: approximately 1.7 x 106 U/mg.
No U.S. standard of potency. Volume after reconstitution with 1 mL diluent is 1 mL.
Before and after reconstitution refrigerate at 2° to 8°C (36° to 46°F). DO NOT FREEZE.
For Subcutaneous Use Only
Amgen® Wyeth®
Manufactured by Immunex Corporation, Thousand Oaks, CA 91320
U.S. License No. 1132
Marketed by Amgen and Wyeth Pharmaceuticals
©2003 Immunex Corporation
Immunex U.S. Patent Numbers: 5,395,760; 5,605,690; 5,945,397; 6,201,105; Re. 36,755

| ENBREL
etanercept solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103795 | 10/06/2005 | |
| ENBREL
etanercept solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103795 | 11/10/2005 | |
| ENBREL
etanercept kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103795 | 01/02/2003 | |
| ENBREL
etanercept solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA103795 | 11/10/2005 | |
| Labeler - Immunex Corp (028134799) |
| Registrant - Amgen, Inc (039976196) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Immunex Rhode Island Corporation | 085509730 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Amgen, Inc | 039976196 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Boehringer Ingelheim Pharma GmbH and Co. KG | 340700520 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Amgen Manufacturing Ltd | 785800020 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| DSM PHARMACEUTICALS INC | 076301910 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Wyeth BioPharma | 174350868 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| BAXTER PHARMACEUTICALS SOLUTIONS LLC | 604719430 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| VETTER PHARMA FERTIGUNG GMBH AND CO KG | 344217323 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| VETTER PHARMA FERTIGUNG GMBH AND CO KG | 316126754 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| VETTER PHARMA FERTIGUNG GMBH AND CO KG | 344069922 | MANUFACTURE, ANALYSIS | |