RIMSO-50
-
dimethyl sulfoxide irrigant
Bioniche Pharma USA LLC
----------
RIMSO-50®brand of dimethyl sulfoxide irrigation, USP
PRESCRIBING INFORMATION
DESCRIPTION
RIMSO-50®, brand of dimethyl sulfoxide (DMSO) 50% w/w Aqueous Solution for intravesical instillation.
Each mL contains 0.54 gm dimethyl sulfoxide STERILE AND NON-PYROGENIC.
Intravesical instillation for the treatment of interstitial cystitis.
NOT FOR I.M. OR I.V. INJECTION.
Rx only.
The active component of RIMSO-50® is dimethyl sulfoxide which has the empirical formula C2H6OS, and is structurally represented as:
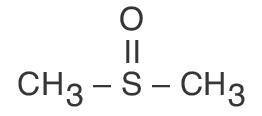
Dimethyl sulfoxide is a clear, colorless and essentially odorless liquid which is miscible with water and most organic solvents. Other physical characteristics include: molecular weight 78.13, melting point 18.3° C, and a specific gravity of 1.096.
CLINICAL PHARMACOLOGY
Dimethyl sulfoxide is metabolised in man by oxidation to dimethyl sulfone or by reduction to dimethyl sulfide. Dimethyl sulfoxide and dimethyl sulfone are excreted in the urine and feces. Dimethyl sulfide is eliminated through the breath and skin and is responsible for the characteristic odor from patients on dimethyl sulfoxide medication. Dimethyl sulfone can persist in serum for longer than two weeks after a single intravesical instillation. No residual accumulation of dimethyl sulfoxide has occurred in man or lower animals who have received treatment for protracted periods of time. Following topical application, dimethyl sulfoxide is absorbed and generally distributed in the tissues and body fluids.
INDICATIONS AND USAGE
RIMSO-50® (dimethyl sulfoxide) is indicated for the symptomatic relief of patients with interstitial cystitis. RIMSO-50® has not been approved as being safe and effective for any other indication. There is no clinical evidence of effectiveness of dimethyl sulfoxide in the treatment of bacterial infections of the urinary tract.
CONTRAINDICATIONS
None known.
WARNINGS
Dimethyl sulfoxide can initiate the liberation of histamine and there has been occasional hypersensitivity reaction with topical administration of dimethyl sulfoxide. This hypersensitivity has been reported in one patients receiving intravesical RIMSO-50®. The physician should be cognizant of this possibility in prescribing RIMSO-50®. If anaphylactoid symptoms develop, appropriate therapy should be instituted.
PRECAUTIONS
Changes in the refractive index and lens opacities have been seen in monkeys, dogs and rabbits given high doses of dimethyl sulfoxide chronically. Since lens changes were noted in animals, full eye evaluations, including slit lamp examinations, are recommended prior to and periodically during treatment.
Approximately every six months patients receiving dimethyl sulfoxide should have a biochemical screening, particularly liver and renal function tests, and complete blood count.
Intravesical instillation of RIMSO-50® may be harmful to patients with urinary tract malignancy because of dimethyl sulfoxide-induced vasodilation.
Some data indicate that dimethyl sulfoxide potentiates other concomitantly administered medications.
Pregnancy Category C
Dimethyl sulfoxide caused teratogenic responses in hamsters, rats and mice when administered intraperitoneally at high doses (2.5 to 12 gm/kg). Oral or topical doses of dimethyl sulfoxide did not cause problems of reproduction in rats, mice and hamsters. Topical doses (5 gm/kg first two days, then 2.5 gm/kg - last eight days) produced terata in rabbits, but in another study, topical doses of 1.1 gm/kg days 3 through 16 of gestation failed to produce any abnormalities. There are no adequate and well controlled studies in pregnant women. Dimethyl sulfoxide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
INFORMATION FOR PATIENTS (Patient Copy)
RIMSO-50® is a sterile solution of 50% dimethyl sulfoxide (DMSO) and 50% water that has been approved by the U.S. Food and Drug Administration for use in the symptomatic relief of patients with interstitial cystitis.
RIMSO-50® will be instilled in the bladder on an inpatient or out-patient basis, which will be determined by your physician.
Some data indicate that dimethyl sulfoxide could change the effectiveness of medication(s) that you may be presently receiving. Be sure to mention the name and dosage of all medicines you are taking to your physician before a RIMSO-50® instillation.
A garlic-like taste may be noted by the patient within a few minutes after instillation of RIMSO-50® (dimethyl sulfoxide). This taste may last several hours. An odor on the breath and skin may be present and remain for up to 72 hours.
Some patients may experience discomfort on administration of the drug. Usually this becomes less prominent with repeated administration.
If you are pregnant or nursing, ask your physician about the advisability of using RIMSO-50®.
Some eye changes have been observed in animals treated with DMSO in large doses for prolonged periods. Therefore your doctor may want you to have eye evaluations, including slit lamp examinations prior to and periodically during treatment.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dimethyl sulfoxide is administered to a nursing woman.
Safety and effectiveness in children have not been established.
Information available to be given to the patient is reprinted at the end of this text.
ADVERSE REACTIONS
A garlic-like taste may be noted by the patient within a few minutes after instillation of RIMSO-50® (dimethyl sulfoxide). This taste may last several hours and because of the presence of metabolites, an odor on the breath and skin may remain for 72 hours.
Transient chemical cystitis has been noted following instillation of dimethyl sulfoxide.
The patient may experience moderately severe discomfort on administration. Usually this becomes less prominent with repeated administration.
DRUG ABUSE AND DEPENDENCE
None known.
OVERDOSAGE
The oral LD50 of dimethyl sulfoxide in the dog is greater than 10 gm/kg. It is improbable that this dosage level could be obtained with intravesical instillation of RIMSO-50® in the patient.
In case of accidental oral ingestion, specific measures should be taken to induce emesis. Additional measures which may be considered are gastric lavage, activated charcoal and force diuresis.
DOSAGE AND ADMINISTRATION
Instillation of 50 mL of RIMSO-50® (dimethyl sulfoxide) directly into the bladder may be accomplished by catheter or asepto syringe and allow to remain for 15 minutes. Application of an analgesic lubricant gel such as lidocaine jelly to the urethra is suggested prior to insertion of the catheter to avoid spasm. The medication is expelled by spontaneous voiding. It is recommended that the treatment be repeated every two weeks until maximum symptomatic relief is obtained. Thereafter, time intervals between therapy may be increased appropriately.
Administration of oral analgesic medication or suppositories containing belladonna and opium prior to the instillation of RIMSO-50® can reduce bladder spasm.
In patients with severe interstitial cystitis with very sensitive bladders, the initial treatment, and possibly the second and third (depending on patient response) should be done under anesthesia. (Saddle block has been suggested).
HOW SUPPLIED
Bottles contain 50 mL of sterile and non-pyrogenic RIMSO-50® (50% w/w dimethyl sulfoxide aqueous solution).
Dimethyl sulfoxide is clear and colorless.
Protect from strong light.
Store at room temperature (59° to 86°F) (15° to 30°C).
NDC #67457-177-50.
For additional information concerning RIMSO-50®, contact Bioniche Pharma USA LLC, Lake Forest, IL 60045
Manufactured for: Bioniche Pharma USA LLC, Lake Forest, IL 60045
Manufactured by: Bioniche Teo., Inverin, Co. Galway, Ireland.
INFORMATION FOR PATIENTS (Physician Copy)
RIMSO-50® is a sterile solution of 50% dimethyl sulfoxide (DMSO) and 50% water that has been approved by the U.S. Food and Drug Administration for use in the symptomatic relief of patients with interstitial cystitis.
RIMSO-50® will be instilled in the bladder on an inpatient or out-patient basis, which will be determined by your physician.
Some data indicate that dimethyl sulfoxide could change the effectiveness of medication(s) that you may be presently receiving. Be sure to mention the name and dosage of all medicines you are taking to your physician before a RIMSO-50® instillation.
A garlic-like taste may be noted by the patient within a few minutes after instillation of RIMSO-50® (dimethyl sulfoxide). This taste may last several hours. An odor on the breath and skin may be present and remain for up to 72 hours.
Some patients may experience discomfort on administration of the drug. Usually this becomes less prominent with repeated administration.
If you are pregnant or nursing, ask your physician about the advisability of using RIMSO-50®.
Some eye changes have been observed in animals treated with DMSO in large doses for prolonged periods. Therefore your doctor may want you to have eye evaluations, including slit lamp examinations prior to and periodically during treatment.
Bioniche Pharma USA LLC,
Lake Forest, IL 60045
0582L100
PRINCIPAL DISPLAY PANEL - 50 mL Vial
RIMSO-50®
brand of
dimethyl sulfoxide
irrigation, USP
STERILE AND NON-PYROGENIC
Rx only.
Manufactured for: Bioniche Pharma USA LLC, Lake Forest, IL 60045
Manufactured by: Bioniche Teo., Inverin, Co. Galway, Ireland.

| RIMSO-50
dimethyl sulfoxide irrigant |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA017788 | 04/01/1978 | |
| Labeler - Bioniche Pharma USA LLC (790384503) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Bioniche Teoranta | 896321325 | MANUFACTURE | |