PULMICORT FLEXHALER
-
budesonide aerosol, powder
AstraZeneca LP
----------
PULMICORT FLEXHALER™ 180 mcg(budesonide inhalation powder, 180 mcg)
PULMICORT FLEXHALER™ 90 mcg
(budesonide inhalation powder, 90 mcg)
For Oral Inhalation Only
DESCRIPTION
Budesonide, the active component of PULMICORT FLEXHALER is a corticosteroid designated chemically as (RS)-11β, 16α, 17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
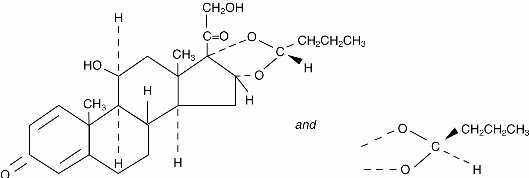
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and in heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 7.4 is 1.6 x 103.
PULMICORT FLEXHALER is an inhalation-driven multi-dose dry powder inhaler containing a formulation of 1 mg per actuation of micronized budesonide and micronized lactose monohydrate which contains trace levels of milk proteins (see CONTRAINDICATIONS and ADVERSE REACTIONS, Adverse Event Reports from Other Sources). Each actuation of PULMICORT FLEXHALER 180 mcg delivers 160 mcg budesonide from the mouthpiece and each actuation of PULMICORT FLEXHALER 90 mcg delivers 80 mcg budesonide from the mouthpiece (based on in vitro testing at 60 L/min for 2 sec). Each PULMICORT FLEXHALER 180 mcg contains 120 actuations and each PULMICORT FLEXHALER 90 mcg contains 60 actuations.
In vitro testing has shown that the dose delivery for PULMICORT FLEXHALER is dependent on airflow through the device, as evidenced by a decrease in the fine particle dose at a flow rate of 30 L/min to a value that is approximately 40-50% of that produced at 60 L/min. At a flow rate of 40 L/min, the fine particle dose is approximately 70% of that produced at 60 L/min. Patient factors such as inspiratory flow rates will also affect the dose delivered to the lungs of patients in actual use (see Patient Information and Instructions for Use). In asthmatic children age 6 to 17 (N=516, FEV1 2.29 [0.97–4.28]) peak inspiratory flow (PIF) through PULMICORT FLEXHALER was 72.5 [19.1 – 103.6] L/min). Inspiratory flows were not measured in the adult pivotal study. Patients should be carefully instructed on the use of this drug product to assure optimal dose delivery.
CLINICAL PHARMACOLOGY
Mechanism of Action
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay.
The activity of PULMICORT FLEXHALER is due to the parent drug, budesonide. In glucocorticoid receptor affinity studies, the 22R form was two times as active as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not interconvert.
The precise mechanism of corticosteroid actions on inflammation in asthma is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of inhibitory activities against multiple cell types (eg, mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (eg, histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic and non-allergic-mediated inflammation. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Studies in asthmatic patients have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects over a wide range of doses from PULMICORT FLEXHALER or inhaled budesonide. This is explained by a combination of a relatively high local anti-inflammatory effect, extensive first pass hepatic degradation of orally absorbed drug (85-95%), and the low potency of formed metabolites (see below).
Pharmacokinetics
Absorption
After oral administration of budesonide, peak plasma concentration was achieved in about 1 to 2 hours and the absolute systemic availability was 6-13%. In contrast, most of budesonide delivered to the lungs is systemically absorbed. In healthy subjects, 34% of the metered dose was deposited in the lungs (as assessed by plasma concentration method and using a different budesonide containing dry-powder inhaler) with an absolute systemic availability of 39% of the metered dose. Peak steady-state plasma concentrations of budesonide delivered from PULMICORT FLEXHALER in adults with asthma (n=39) occurred at approximately 10 minutes post-dose and averaged 0.6 and 1.6 nmol/L at doses of 180 mcg once daily and 360 mcg twice daily, respectively.
In asthmatic patients, budesonide showed a linear increase in AUC and Cmax with increasing dose after both a single dose and repeated dosing of inhaled budesonide.
Distribution
The volume of distribution of budesonide was approximately 3 L/kg. It was 85-90% bound to plasma proteins. Protein binding was constant over the concentration range (1-100 nmol/L) achieved with, and exceeding, recommended doses of PULMICORT FLEXHALER. Budesonide showed little or no binding to corticosteroid binding globulin. Budesonide rapidly equilibrated with red blood cells in a concentration independent manner with a blood/plasma ratio of about 0.8.
Metabolism
In vitro studies with human liver homogenates have shown that budesonide is rapidly and extensively metabolized. Two major metabolites formed via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4) catalyzed biotransformation have been isolated and identified as 16α-hydroxyprednisolone and 6β-hydroxybudesonide. The corticosteroid activity of each of these two metabolites is less than 1% of that of the parent compound. No qualitative differences between the in vitro and in vivo metabolic patterns have been detected. Negligible metabolic inactivation was observed in human lung and serum preparations.
Excretion/Elimination
The 22R form of budesonide was preferentially cleared by the liver with systemic clearance of 1.4 L/min vs. 1.0 L/min for the 22S form. The terminal half-life, 2 to 3 hours, was the same for both epimers and was independent of dose. Budesonide was excreted in urine and feces in the form of metabolites. Approximately 60% of an intravenous radiolabeled dose was recovered in the urine. No unchanged budesonide was detected in the urine.
Special Populations
No clinically relevant pharmacokinetic differences have been identified due to race, sex, or advanced age.
Pediatric
Following intravenous dosing in pediatric patients age 10-14 years, plasma half-life was shorter than in adults (1.5 hours vs. 2.0 hours in adults). In the same population following inhalation of budesonide via a pressurized metered-dose inhaler, absolute systemic availability was similar to that in adults.
Peak steady-state plasma concentrations of budesonide delivered via PULMICORT FLEXHALER in children and adolescents with asthma (n=14) occurred at approximately 15 to 30 minutes post-dose and averaged 0.4 and 1.5 nmol/L at doses of 180 mcg once daily and 360 mcg twice daily, respectively.
Hepatic Insufficiency
Reduced liver function may affect the elimination of corticosteroids. The pharmacokinetics of budesonide were affected by compromised liver function as evidenced by a doubled systemic availability after oral ingestion. The intravenous pharmacokinetics of budesonide were, however, similar in cirrhotic patients and in healthy subjects.
Nursing Mothers
The disposition of budesonide when delivered by inhalation from a dry powder inhaler at doses of 200 or 400 mcg twice daily for at least 3 months was studied in eight lactating women with asthma from 1 to 6 months postpartum. Systemic exposure to budesonide in these women appears to be comparable to that in non-lactating women with asthma from other studies. Breast milk obtained over eight hours post-dose revealed that the maximum concentration of budesonide for the 400 and 800 mcg doses was 0.39 and 0.78 nmol/L, respectively, and occurred within 45 minutes after dosing. The estimated oral daily dose of budesonide from breast milk to the infant is approximately 0.007 and 0.014 mcg/kg/day for the two dose regimens used in this study, which represents approximately 0.3% to 1% of the dose inhaled by the mother. Budesonide levels in plasma samples obtained from five infants at about 90 minutes after breastfeeding (and about 140 minutes after drug administration to the mother) were below quantifiable levels (<0.02 nmol/L in four infants and <0.04 nmol/L in one infant) (see PRECAUTIONS, Nursing Mothers).
Drug-Drug Interactions
Ketoconazole, a potent inhibitor of cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4), the main metabolic enzyme for corticosteroids, increased plasma levels of orally ingested budesonide. At recommended doses, cimetidine had a slight but clinically insignificant effect on the pharmacokinetics of oral budesonide. For more information, please see PRECAUTIONS, Drug Interactions.
Pharmacodynamics
To confirm that systemic absorption is not a significant factor in the clinical efficacy of inhaled budesonide, a clinical study in patients with asthma was performed comparing 400 mcg budesonide administered via a pressurized metered-dose inhaler with a tube spacer to 1400 mcg of oral budesonide and placebo. The study demonstrated the efficacy of inhaled budesonide but not orally ingested budesonide, despite comparable systemic levels. Thus, the therapeutic effect of conventional doses of orally inhaled budesonide are largely explained by its direct action on the respiratory tract.
Generally, budesonide has a relatively rapid onset of action for an inhaled corticosteroid. Improvement in asthma control following inhalation of budesonide can occur within 24 hours of beginning treatment although maximum benefit may not be achieved for 1 to 2 weeks, or longer.
Inhaled budesonide has been shown to decrease airway reactivity in various challenge models, including histamine, methacholine, sodium metabisulfite, and adenosine monophosphate in patients with hyperreactive airways. The clinical relevance of these models is not certain.
Pretreatment with inhaled budesonide 1600 mcg daily (800 mcg twice daily) for 2 weeks reduced the acute (early-phase reaction) and delayed (late-phase reaction) decrease in FEV1 following inhaled allergen challenge.
The effects of inhaled budesonide on the hypothalamic-pituitary-adrenal (HPA) axis were studied in 905 adults and 404 pediatric patients with asthma. For most patients, the ability to increase cortisol production in response to stress, as assessed by cosyntropin (ACTH) stimulation test, remained intact with inhaled budesonide treatment at recommended doses. For adult patients treated with 100, 200, 400, or 800 mcg twice daily for 12 weeks, 4%, 2%, 6%, and 13% respectively, had an abnormal stimulated cortisol response (peak cortisol <14.5 mcg/dL assessed by liquid chromatography following short-cosyntropin test) as compared with 8% of patients treated with placebo. Similar results were obtained in pediatric patients. In another study in adults, doses of 400, 800 and 1600 mcg of inhaled budesonide twice daily for 6 weeks were examined; 1600 mcg twice daily (twice the maximum recommended dose) resulted in a 27% reduction in stimulated cortisol (6-hour ACTH infusion) while 10 mg prednisone resulted in a 35% reduction. In this study, no patient taking doses of 400 and 800 mcg twice daily met the criterion for an abnormal stimulated cortisol response (peak cortisol <14.5 mcg/dL assessed by liquid chromatography) following ACTH infusion. An open-label, long-term follow-up of 1133 patients for up to 52 weeks confirmed the minimal effect on the HPA axis (both basal and stimulated plasma cortisol) of inhaled budesonide when administered at doses ranging from 100 to 800 mcg twice daily. In patients who had previously been oral steroid-dependent, use of inhaled budesonide at doses ranging from 100 to 800 mcg twice daily was associated with higher stimulated cortisol response compared with baseline following 1 year of therapy.
The administration of inhaled budesonide via a different dry-powder inhaler in doses up to 800 mcg/day (mean daily dose 445 mcg/day) or via a pressurized metered-dose inhaler in doses up to 1200 mcg/day (mean daily dose 620 mcg/day) to 216 pediatric patients (age 3 to 11 years) for 2 to 6 years had no significant effect on statural growth compared with non-corticosteroid therapy in 62 matched control patients. However, the long-term effect of inhaled budesonide on growth is not fully known.
Clinical Studies
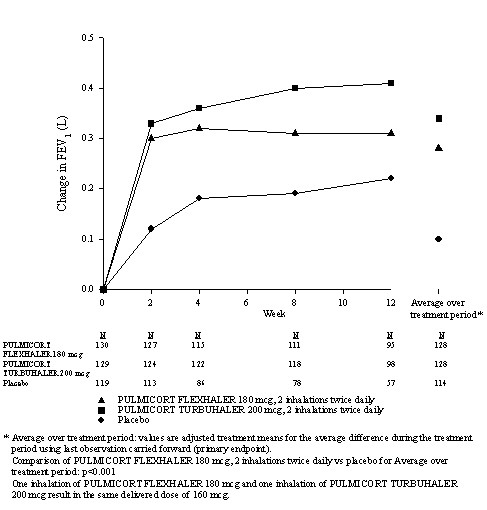
The safety and efficacy of PULMICORT FLEXHALER were evaluated in two 12-week, double-blind, randomized, parallel-group, placebo-controlled clinical studies conducted at sites in the United States and Asia involving 1137 patients aged 6 to 80 years with mild to moderate asthma. Study 1 evaluated PULMICORT FLEXHALER 180 mcg, PULMICORT TURBUHALER® 200 mcg, and placebo, each administered as 1 inhalation once daily or 2 inhalations twice daily in patients 18 years of age and older with mild to moderate asthma previously treated with inhaled corticosteroids. The delivered dose of PULMICORT FLEXHALER 180 mcg and PULMICORT TURBUHALER 200 mcg are the same; each delivers 160 mcg from the mouthpiece. Study 2 evaluated PULMICORT FLEXHALER 90 mcg, 2 inhalations once daily or 4 inhalations twice daily, PULMICORT TURBUHALER 200 mcg, 1 inhalation once daily or 2 inhalations twice daily, and placebo in pediatric patients aged 6 to 17 years with mild to moderate asthma. Both of the studies had a 2-week placebo treatment run-in period followed by a 12-week randomized treatment period. The primary endpoint was the difference between baseline and the mean of the treatment-period FEV1 (adults) or FEV1 % predicted (children).
Adult Patients with Asthma (Study 1)
This study enrolled 621 patients aged ≥18 to 80 years with mild-to-moderate asthma (mean baseline % predicted FEV1 64.3%) whose symptoms were previously controlled on inhaled corticosteroids. Mean change from baseline in FEV1 in the PULMICORT FLEXHALER 180 mcg, 2 inhalations twice-daily group was 0.28 liters, as compared to 0.10 liters in the placebo group (p<0.001). Secondary endpoints of morning and evening peak expiratory flow rate, daytime asthma symptom severity, nighttime asthma symptom severity, daily rescue medication use, and the percentage of patients who met predefined asthma related withdrawal criteria showed differences from baseline favoring PULMICORT FLEXHALER over placebo. The responses of PULMICORT FLEXHALER compared with PULMICORT TURBUHALER tended to be lower.
12-Week Trial in Adult Patients with Mild to Moderate Asthma (Study 1)
Mean Change from Baseline in FEV1 (L)
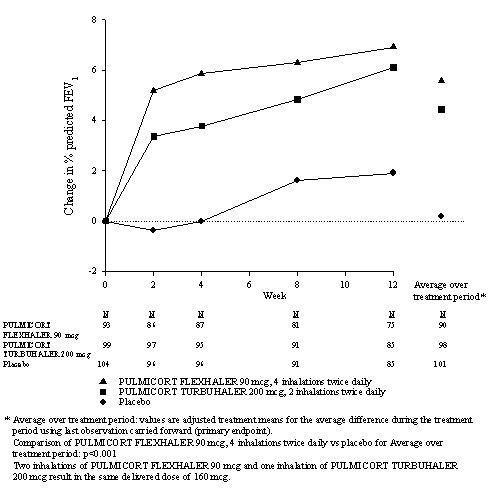
Pediatric and Adolescent Patients with Asthma (Study 2)
This study enrolled 516 patients aged 6 to 17 years with mild asthma (mean baseline % predicted FEV1 84.9%). The study population included patients previously treated with inhaled corticosteroids for no more than 30 days before the study began (4%) and patients who were naïve to inhaled corticosteroids (96%). Mean change from baseline in % predicted FEV1 during the 12-week treatment period in the PULMICORT FLEXHALER 90 mcg, 4 inhalations twice daily treatment group was 5.6 compared with 0.2 in the placebo group (p<0.001). Secondary endpoints of morning and evening PEF showed differences from baseline favoring PULMICORT FLEXHALER over placebo.
12-Week Trial in Pediatric Patients With Mild Asthma (Study 2)
Mean Change from Baseline in Percent Predicted FEV1
INDICATIONS AND USAGE
PULMICORT FLEXHALER is indicated for the maintenance treatment of asthma as prophylactic therapy in adult and pediatric patients six years of age or older. It is also indicated for patients requiring oral corticosteroid therapy for asthma. Many of those patients may be able to reduce or eliminate their requirement for oral corticosteroids over time.
PULMICORT FLEXHALER is NOT indicated for the relief of acute bronchospasm.
CONTRAINDICATIONS
PULMICORT FLEXHALER is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
PULMICORT FLEXHALER is contraindicated in patients with hypersensitivity to any of its ingredients (see DESCRIPTION and ADVERSE REACTIONS, Adverse Event Reports from Other Sources).
WARNINGS
Particular care is needed for patients who are transferred from systemically active corticosteroids to PULMICORT FLEXHALER because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although PULMICORT FLEXHALER may provide control of asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a medical identification card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to PULMICORT FLEXHALER. Lung function (FEV1 or AM PEF), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to PULMICORT FLEXHALER may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy, eg, rhinitis, conjunctivitis, arthritis, eosinophilic conditions, and eczema.
Patients who are on drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG), as appropriate, may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chicken pox develops, treatment with antiviral agents may be considered. The immune responsiveness to varicella vaccine was evaluated in pediatric patients with asthma ages 12 months to 8 years with budesonide inhalation suspension. (see PRECAUTIONS, Drug Interactions).
PULMICORT FLEXHALER is not a bronchodilator and is not indicated for rapid relief of bronchospasm or other acute episodes of asthma.
As with other inhaled asthma medications, bronchospasm, with an immediate increase in wheezing, may occur after dosing. If bronchospasm occurs following dosing with PULMICORT FLEXHALER, it should be treated immediately with a fast-acting inhaled bronchodilator. Treatment with PULMICORT FLEXHALER should be discontinued and alternate therapy instituted.
Patients should be instructed to contact their physician immediately when episodes of asthma not responsive to their usual doses of bronchodilators occur during treatment with PULMICORT FLEXHALER. During such episodes, patients may require therapy with oral corticosteroids.
PRECAUTIONS
General
PULMICORT FLEXHALER contains small amounts of lactose, which contains trace levels of milk proteins. It is possible that cough, wheezing, or bronchospasm may occur in patients who have a severe milk protein allergy (see CONTRAINDICATIONS and ADVERSE REACTIONS, Adverse Event Reports from Other Sources).
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal, eg, joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function (see DOSAGE AND ADMINISTRATION).
In responsive patients, PULMICORT FLEXHALER may permit control of asthma symptoms with less suppression of HPA-axis function than therapeutically equivalent oral doses of prednisone. Since budesonide is absorbed into the circulation and can be systemically active, the beneficial effects of PULMICORT FLEXHALER in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing PULMICORT FLEXHALER.
Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with PULMICORT FLEXHALER should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism, reduced bone mineral density, and adrenal suppression may appear in a small number of patients, particularly at higher doses. If such changes occur, PULMICORT FLEXHALER should be reduced slowly, consistent with accepted procedures for management of asthma symptoms and for tapering of systemic steroids.
Orally inhaled corticosteroids, including budesonide, may cause a reduction in growth velocity when administered to pediatric patients. A reduction in growth velocity may occur as a result of inadequate control of asthma or from use of corticosteroids for treatment. The potential effects of prolonged treatment on growth velocity should be weighed against the clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including PULMICORT FLEXHALER, each patient should be titrated to his/her lowest effective dose (see PRECAUTIONS, Pediatric Use).
Although patients in clinical trials have received inhaled budesonide on a continuous basis for periods of 1 to 2 years, the long-term local and systemic effects of PULMICORT FLEXHALER in human subjects are not completely known. In particular, the effects resulting from chronic use of PULMICORT FLEXHALER on developmental or immunological processes in the mouth, pharynx, trachea, and lung are unknown.
In clinical trials with PULMICORT FLEXHALER, localized infections with Candida albicans occurred in the mouth and pharynx in some patients. These infections may require treatment with appropriate antifungal therapy and/or discontinuance of treatment with PULMICORT FLEXHALER.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract, untreated systemic fungal, bacterial, viral or parasitic infections, or ocular herpes simplex.
Rare instances of glaucoma, increased intraocular pressure, and cataracts have been reported following the inhaled administration of corticosteroids.
Information for Patients
Patients being treated with PULMICORT FLEXHALER should receive the following information and instructions. This information is intended to aid the patient in the safe and effective use of the medication. It is not a disclosure of all possible adverse or intended effects. For proper use of PULMICORT FLEXHALER and to attain maximum improvement, the patient should read and follow the accompanying Patient Information and Instructions for Use.
-
Patients should use PULMICORT FLEXHALER at regular intervals as directed since its effectiveness depends on regular use. The patient should not alter the prescribed dosage unless advised to do so by the physician.
-
Patients should be advised that PULMICORT FLEXHALER is not a bronchodilator and is not intended to treat acute or life-threatening episodes of asthma.
-
Patients should be advised that the effectiveness of PULMICORT FLEXHALER depends on proper use of the device and inhalation-administering technique:
-
PULMICORT FLEXHALER must be in the upright position (mouthpiece on top) during loading in order to provide the correct dose.
-
PULMICORT FLEXHALER must be primed before the unit is used for the very first time. To prime your PULMICORT FLEXHALER, follow the steps below:
-
Hold the unit so that the white cover points upward, grasp the inhaler by the brown grip in one hand and with the other hand, turn the white cover and lift it off.
-
Hold PULMICORT FLEXHALER by the brown grip, in the upright position (with mouthpiece up) in one hand. Using the thumb and index finger of your other hand, grasp the inhaler in the middle. Do not hold the inhaler at the top of the mouthpiece.
-
Twist the brown grip as far as it will go in one direction and then fully back again in the other direction until it stops (it does not matter which way you turn it first). You will hear a click during one of the twisting movements.
-
Repeat Step 3. Your PULMICORT FLEXHALER is now primed.
-
-
To load the first dose, twist the brown grip fully in one direction as far as it will go and then fully back again in the other direction as far as it will go until it clicks. It does not matter which way you turn it first.
-
PULMICORT FLEXHALER is designed to deliver only one dose at a time, no matter how often you click the brown grip, although the dose indicator will continue to advance. If you accidentally blow into your inhaler after loading a dose, simply follow the instructions for loading a new dose.
-
Patients should be advised not to shake the inhaler.
-
Patients should place the mouthpiece between the lips and inhale forcefully and deeply. The powder is then delivered to the lungs.
-
Patients should not exhale through PULMICORT FLEXHALER.
-
Due to the small volume of powder, patients may not sense the presence of any medication entering the lungs when inhaling from PULMICORT FLEXHALER. This lack of sensation does not indicate that the patient is not receiving benefit from PULMICORT FLEXHALER.
-
Patients should be advised that rinsing the mouth with water without swallowing after each dosing may decrease the risk of the development of oral candidiasis.
-
Patients should be instructed that they will receive a new PULMICORT FLEXHALER unit each time they refill their prescription. Patients should be advised to discard the whole device after the labeled number of inhalations has been used even though it may not feel completely empty and may continue to operate. The number in the middle of the dose indicator window tells how many doses are left in the inhaler. The inhaler is empty when the number zero (“0”) on the red background reaches the middle of the window. You may still hear a sound if you shake it — this sound is not the medicine. This sound is produced by the drying agent inside.
-
The dose indicator is connected to the turning grip and moves (counts down) every time a dose is loaded. It is unlikely that you will see the dose indicator move with each individual dose. Advancement of the indicator can be seen after intervals of 5 or so doses.
-
The dose indicator starts with either the number 60 or 120 when full, depending upon the strength of the product. The indicator is marked in intervals of 10 doses, alternating numbers and dashes, down to “0”.
-
If the instructions for loading the dose are completed more than once prior to inhaling the dose, only one dose will be received. However, the dose indicator will advance slightly but it is unlikely that you will see the dose indicator move with each individual dose.
-
The grip will still twist and “click” even when your inhaler is empty.
-
PULMICORT FLEXHALER should not be used with a spacer.
-
The mouthpiece should not be bitten or chewed.
-
Replace the cover securely after each opening.
-
Patients should keep PULMICORT FLEXHALER clean and dry at all times.
-
Patients should be advised that improvement in asthma control following inhalation of budesonide can occur within 24 hours of beginning treatment although maximum benefit may not be achieved for 1 to 2 weeks, or longer. If symptoms do not improve in that time frame, or if the condition worsens, the patient should be instructed not to increase the dosage, but to contact the physician.
-
Patients whose systemic corticosteroids have been reduced or withdrawn should be instructed to carry a warning card indicating that they may need supplemental systemic corticosteroids during periods of stress or an asthma attack that does not respond to bronchodilators.
-
Patients should be advised not to stop the use of PULMICORT FLEXHALER abruptly.
-
Patients should be warned to avoid exposure to chicken pox or measles and if they are exposed, to consult their physicians without delay.
-
Long-term use of inhaled corticosteroids, including budesonide, may increase the risk of some eye problems (cataracts or glaucoma). Regular eye examinations should be considered.
-
Women considering the use of PULMICORT FLEXHALER should consult with their physician if they are pregnant or intend to become pregnant, or if they are breast-feeding a baby.
-
Patients considering use of PULMICORT FLEXHALER should consult with their physician if they are allergic to budesonide, milk proteins or any other orally inhaled corticosteroid.
-
PULMICORT FLEXHALER contains small amounts of lactose, which contains trace levels of milk proteins. It is possible that cough, wheezing, or bronchospasm may occur in patients who have a severe milk protein allergy. (see CONTRAINDICATIONS and ADVERSE REACTIONS, Adverse Event Reports from Other Sources).
-
Patients should inform their physician of other medications they are taking as PULMICORT FLEXHALER may not be suitable in some circumstances and the physician may wish to use a different medicine.
Drug Interactions
In clinical studies, concurrent administration of budesonide and other drugs commonly used in the treatment of asthma has not resulted in an increased frequency of adverse events. The main route of metabolism of budesonide, as well as other corticosteroids, is via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4). After oral administration of ketoconazole, a potent inhibitor of CYP3A4, the mean plasma concentration of orally administered budesonide increased. Concomitant administration of other known inhibitors of CYP3A4 (eg, itraconazole, clarithromycin, erythromycin, etc.) may inhibit the metabolism of, and increase the systemic exposure to, budesonide. Care should be exercised when budesonide is coadministered with long-term ketoconazole and other known CYP3A4 inhibitors.
Varicella Vaccine: An open-label, nonrandomized clinical study examined the immune responsiveness to varicella vaccine in 243 asthma patients 12 months to 8 years of age who were treated with budesonide inhalation suspension 0.25 mg to 1 mg daily (n=151) or noncorticosteroid asthma therapy (n=92) (i.e., beta2-agonists, leukotriene receptor antagonists, cromones). The percentage of patients developing a seroprotective antibody titer of ≥5.0 (gpELISA value) in response to the vaccination was similar in patients treated with budesonide inhalation suspension (85%), compared to patients treated with noncorticosteroid asthma therapy (90%). No patient treated with budesonide inhalation suspension developed chicken pox as a result of vaccination.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies were conducted in rats and mice using oral administration to evaluate the carcinogenic potential of budesonide.
In a 104-week oral study in Sprague-Dawley rats, a statistically significant increase in the incidence of gliomas was observed in male rats receiving an oral dose of 50 mcg/kg/day (less than the maximum recommended daily inhalation dose in adults and children on a mcg/m2 basis). No tumorigenicity was seen in male and female rats at respective oral doses up to 25 and 50 mcg/kg (less than the maximum recommended daily inhalation dose in adults and children on a mcg/m2 basis). In two additional two-year studies in male Fischer and Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (less than the maximum recommended daily inhalation dose in adults and children on a mcg/m2 basis). However, in the male Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (less than the maximum recommended daily inhalation dose in adults and children on a mcg/m2 basis). The concurrent reference corticosteroids (prednisone and triamcinolone acetonide) in these two studies showed similar findings.
There was no evidence of a carcinogenic effect when budesonide was administered orally for 91 weeks to mice at doses up to 200 mcg/kg/day (less than the maximum recommended daily inhalation dose in adults and children on a mcg/m2 basis).
Budesonide was not mutagenic or clastogenic in six different test systems: Ames Salmonella/microsome plate test, mouse micronucleus test, mouse lymphoma test, chromosome aberration test in human lymphocytes, sex-linked recessive lethal test in Drosophila melanogaster, and DNA repair analysis in rat hepatocyte culture.
In rats, budesonide had no effect on fertility at subcutaneous doses up to 80 mcg/kg (less than the maximum recommended human daily inhalation dose on a mcg/m2 basis).
At 20 mcg/kg/day (less than the maximum recommended human daily inhalation dose on a mcg/m2 basis), decreases in maternal body weight gain, prenatal viability, and viability of the young at birth and during lactation were observed. No such effects were noted at 5 mcg/kg (less than the maximum recommended human daily inhalation dose in adults on a mcg/m2 basis).
Pregnancy
Teratogenic Effects: Pregnancy category B
As with other glucocorticoids, budesonide produced fetal loss, decreased pup weight, and skeletal abnormalities at subcutaneous doses of 25 mcg/kg/day in rabbits (less than the maximum recommended human daily inhalation dose on a mcg/m2 basis) and 500 mcg/kg/day in rats (approximately 3 times the maximum recommended human daily inhalation dose on a mcg/m2 basis). No teratogenic or embryocidal effects were observed in rats when budesonide was administered by inhalation at doses up to 250 mcg/kg/day (equivalent to the maximum recommended human daily inhalation dose on a mcg/m2 basis).
Experience with oral corticosteroids since their introduction in pharmacologic as opposed to physiologic doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans.
Studies of pregnant women, however, have not shown that inhaled budesonide increases the risk of abnormalities when administered during pregnancy. The results from a large population-based prospective cohort epidemiological study reviewing data from three Swedish registries covering approximately 99% of the pregnancies from 1995-1997 (i.e., Swedish Medical Birth Registry; Registry of Congenital Malformations; Child Cardiology Registry) indicate no increased risk for congenital malformations from the use of inhaled budesonide during early pregnancy. Congenital malformations were studied in 2,014 infants born to mothers reporting the use of inhaled budesonide for asthma in early pregnancy (usually 10-12 weeks after the last menstrual period), the period when most major organ malformations occur. The rate of recorded congenital malformations was similar compared with the general population rate (3.8 % vs. 3.5%, respectively). In addition, after exposure to inhaled budesonide, the number of infants born with orofacial clefts was similar to the expected number in the normal population (4 children vs. 3.3, respectively).
These same data were utilized in a second study bringing the total to 2,534 infants whose mothers were exposed to inhaled budesonide. In this study, the rate of congenital malformations among infants whose mothers were exposed to inhaled budesonide during early pregnancy was not different from the rate for all newborn babies during the same period (3.6%).
Despite the animal findings, it would appear that the possibility of fetal harm is remote if the drug is used during pregnancy. Nevertheless, because the studies in humans cannot rule out the possibility of harm, PULMICORT FLEXHALER should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully observed.
Nursing Mothers
Budesonide, like other corticosteroids, is secreted in human milk. Data with budesonide delivered via dry powder inhaler indicates that the total daily oral dose of budesonide available in breast milk to the infant is approximately 0.3% to 1% of the dose inhaled by the mother (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations, Nursing Mothers). No studies have been conducted in breastfeeding women specifically with PULMICORT FLEXHALER; however, the dose of budesonide available to the infant in breast milk, as a percentage of the maternal dose, would be expected to be similar. PULMICORT FLEXHALER should be used in nursing women only if clinically appropriate. Prescribers should weigh the known benefits of breastfeeding for the mother and the infant against the potential risks of minimal budesonide exposure in the infant. Dosing considerations include prescription or titration to the lowest clinically effective dose and use of PULMICORT FLEXHALER immediately after breastfeeding to maximize the time interval between dosing and breastfeeding to minimize infant exposure. However, in general, PULMICORT FLEXHALER use should not delay or interfere with infant feeding.
Pediatric Use
Safety and effectiveness of PULMICORT FLEXHALER in pediatric patients below 6 years of age have not been established.
Clinical studies with inhaled budesonide included 704 patients 6 to 17 years of age (n=204 treated with PULMICORT FLEXHALER). The frequency of adverse events observed with PULMICORT FLEXHALER in pediatric patients 6 to 17 years of age was similar to that of patients 18 to 80 years of age.
Controlled clinical studies have shown that orally inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids including the impact on final adult height are unknown. The potential for “catch up” growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied.
In a study of asthmatic children 5-12 years of age, those treated with PULMICORT TURBUHALER 200 mcg twice daily (n=311) had a 1.1-centimeter reduction in growth compared with those receiving placebo (n=418) at the end of one year; the difference between these two treatment groups did not increase further over three years of additional treatment. By the end of four years, children treated with PULMICORT TURBUHALER and children treated with placebo had similar growth velocities. Conclusions drawn from this study may be confounded by the unequal use of corticosteroids in the treatment groups and inclusion of data from patients attaining puberty during the course of the study.
The growth of pediatric patients receiving orally inhaled corticosteroids, including PULMICORT FLEXHALER, should be monitored routinely (eg, via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks and benefits associated with alternative therapies. To minimize the systemic effects of inhaled corticosteroids, including PULMICORT FLEXHALER, each patient should be titrated to his/her lowest effective dose.
Geriatric Use
Of the total number of patients in controlled clinical studies receiving inhaled budesonide, 153 (n=11 treated with PULMICORT FLEXHALER ) were 65 years of age or older and one was age 75 years or older. No overall differences in safety were observed between these patients and younger patients. Clinical studies did not include sufficient numbers of patients aged 65 years and over to determine differences in efficacy between elderly and younger patients. Other reported clinical or medical surveillance experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The following adverse reactions were reported in patients treated with PULMICORT FLEXHALER 180 or 90 mcg in two double-blind, placebo-controlled clinical trials in which 226 patients age 6-80 years, previously receiving bronchodilators, inhaled corticosteroids, or both, were treated with PULMICORT FLEXHALER, administered as 360 mcg twice daily for 12 weeks.
The following table shows the incidence of adverse events (whether considered drug-related or non-drug-related by the investigators) that occurred at a rate of ≥1% in the PULMICORT FLEXHALER group and were more common than in the placebo group.
| Adverse Event | PULMICORT FLEXHALER 360 mcg twice daily N=226 % | Placebo N=230 % |
|---|---|---|
|
Nasopharyngitis |
9.3 |
8.3 |
|
Nasal congestion |
2.7 |
0.4 |
|
Pharyngitis |
2.7 |
1.7 |
|
Rhinitis allergic |
2.2 |
1.3 |
|
Viral upper respiratory tract infection |
2.2 |
1.3 |
|
Nausea |
1.8 |
0.9 |
|
Viral gastroenteritis |
1.8 |
0.4 |
|
Otitis media |
1.3 |
0.9 |
|
Oral candidiasis |
1.3 |
0.4 |
|
Average exposure duration (days) |
76.2 |
68.2 |
Long-Term Safety
Non-placebo controlled long-term studies in children (at doses up to 360 mcg daily), and adolescent and adult subjects (at doses up to 720 mcg daily), treated for up to one year with PULMICORT FLEXHALER, revealed a similar pattern and incidence of adverse events.
Adverse Event Reports from Other Sources
The following other adverse events occurred in placebo-controlled clinical trials with similar or lower budesonide doses with PULMICORT TURBUHALER with an incidence of ≥1% in the budesonide group and were more common than in the placebo group:
≥3%: respiratory infection, sinusitis, headache, pain, back pain, fever.
≥1-3%: neck pain, syncope, abdominal pain, dry mouth, vomiting, weight gain, fracture, myalgia, hypertonia, migraine, ecchymosis, insomnia, infection, taste perversion, voice alteration.
Higher doses of PULMICORT TURBUHALER 800 mcg twice daily resulted in an increased incidence of voice alteration, flu syndrome, dyspepsia, gastroenteritis, nausea, and back pain, compared with doses of 400 mcg twice daily.
In a 20-week trial in adult asthmatics who previously required oral corticosteroids, the effects of inhaled budesonide with PULMICORT TURBUHALER 400 mcg twice daily (N=53) and 800 mcg twice daily (N=53) were compared with placebo (N=53) on the frequency of reported adverse events. In considering these data, the increased average duration of exposure for inhaled budesonide patients (78 days for inhaled budesonide vs. 41 days for placebo) should be taken into account. Adverse events, whether considered drug-related or non-drug-related by the investigators, reported in more than five patients in the budesonide group and which occurred more frequently with budesonide than placebo are given (% inhaled budesonide and % placebo): asthenia (9% and 2%), headache (12% and 2%), pain (10% and 2%), dyspepsia (8% and 0%), nausea (6% and 0%), oral candidiasis (10% and 0%), arthralgia (6% and 0%), cough increased (6% and 2%), respiratory infection (32% and 13%), rhinitis (6% and 2%), sinusitis (16% and 11%).
Rare adverse events reported in the published literature or from worldwide marketing experience with any formulation of inhaled budesonide include: immediate and delayed hypersensitivity reactions including rash, contact dermatitis, urticaria, angioedema and bronchospasm; symptoms of hypocorticism and hypercorticism; glaucoma, cataracts; psychiatric symptoms including depression, aggressive reactions, irritability, anxiety and psychosis.
Post-marketing experience with PULMICORT FLEXHALER includes: very rare cough, wheezing, or bronchospasm in patients with severe milk protein hypersensitivity.
OVERDOSAGE
The potential for acute toxic effects following overdose of PULMICORT FLEXHALER is low. If used at excessive doses for prolonged periods, systemic corticosteroid effects such as hypercorticism may occur (see PRECAUTIONS). Another budesonide-containing dry powder inhaler at 3200 mcg daily administered for 6 weeks caused a significant reduction (27%) in the plasma cortisol response to a 6-hour infusion of ACTH compared with placebo (+1%). The corresponding effect of 10 mg prednisone daily was a 35% reduction in the plasma cortisol response to ACTH.
The minimal inhalation lethal dose in mice was 100 mg/kg (approximately 280 times the maximum recommended daily inhalation dose in adults and approximately 330 times the maximum recommended daily inhalation dose in children on a mcg/m2 basis). There were no deaths following the administration of an inhalation dose of 68 mg/kg in rats (approximately 380 times the maximum recommended daily inhalation dose in adults and approximately 450 times the maximum recommended daily inhalation dose in children on a mcg/m2 basis). The minimal oral lethal dose was 200 mg/kg in mice (approximately 560 times the maximum recommended daily inhalation dose in adults and approximately 670 times the maximum recommended daily inhalation dose in children on a mcg/m2 basis) and less than 100 mg/kg in rats (approximately 560 times the maximum recommended daily inhalation dose in adults and approximately 670 times the maximum recommended daily inhalation dose in children based on a mcg/m2 basis).
Post-marketing experience showed that acute overdose of inhaled budesonide commonly remained asymptomatic. The use of excessive doses (up to 6400 mcg daily) for prolonged periods showed systemic corticosteroid effects such as hypercorticism.
DOSAGE AND ADMINISTRATION
PULMICORT FLEXHALER should be administered by the orally inhaled route in asthmatic patients age 6 years and older. Individual patients will experience a variable onset and degree of symptom relief. Generally, budesonide has a relatively rapid onset of action for an inhaled corticosteroid. Improvement in asthma control following inhaled administration of budesonide can occur within 24 hours of initiation of treatment, although maximum benefit may not be achieved for 1 to 2 weeks, or longer. The safety and efficacy of PULMICORT FLEXHALER when administered in excess of recommended doses have not been established.
A definitive comparative therapeutic ratio between PULMICORT FLEXHALER and PULMICORT TURBUHALER has not been established. For patients who have been on PULMICORT TURBUHALER the dose of PULMICORT FLEXHALER may not be predicted by the dose of that product. The clinical response of PULMICORT FLEXHALER compared with PULMICORT TURBUHALER tends to be lower (see Clinical Studies). Any patient who is switched from PULMICORT TURBUHALER to PULMICORT FLEXHALER should be dosed appropriately, taking into account the dosing recommendations, and titrating the dose as dictated by the clinical response.
Adults (age 18 and older): The recommended starting dosage is 360 mcg twice daily. In some patients, a starting dosage of 180 mcg twice daily may be adequate. The maximum dosage should not exceed 720 mcg twice daily.
Children (age 6 to 17): The recommended starting dosage is 180 mcg twice daily. In some patients a starting dosage of 360 mcg twice daily may be appropriate. The maximum dosage should not exceed 360 mcg twice daily.
Dose Titration
As with any inhaled corticosteroid, physicians are advised to select the dosage of PULMICORT FLEXHALER that would be appropriate based upon the patient’s disease severity and titrate the dosage of PULMICORT FLEXHALER downward over time to the lowest level that maintains proper asthma control. In adult patients who are well controlled, a dosage of 180 mcg twice daily may be considered. In some adult patients, a starting dosage of 180 mcg twice daily may be adequate. If the 180 mcg twice daily dosage of PULMICORT FLEXHALER in adults does not provide adequate control, the dosage should be increased.
Patients Maintained on Chronic Oral Corticosteroids
Clinical studies with PULMICORT FLEXHALER did not evaluate patients on oral corticosteroids. However, clinical studies with therapeutic doses of PULMICORT TURBUHALER did show efficacy in the management of asthmatics dependent or maintained on systemic corticosteroids. If a patient is already on a systemic corticosteroid for asthma control, PULMICORT FLEXHALER should be used concurrently with the patient’s usual maintenance dose of systemic corticosteroid. The patient’s asthma should be reasonably stable before withdrawal of oral corticosteroids is initiated. After approximately one week, gradual withdrawal of the systemic corticosteroid may be started by reducing the daily or alternate daily dose. The next reduction is made after an interval of one or two weeks, depending on the response of the patient. Generally, these decrements should not exceed 2.5 mg of prednisone or its equivalent. A slow rate of withdrawal is strongly recommended. During reduction of oral corticosteroids, patients should be carefully monitored for asthma instability, including objective measures of airway function, and for adrenal insufficiency (see WARNINGS). During withdrawal, some patients may experience symptoms of systemic corticosteroid withdrawal, eg, joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement in pulmonary function. Such patients should be encouraged to continue with PULMICORT FLEXHALER but should be monitored for objective signs of adrenal insufficiency. If evidence of adrenal insufficiency occurs, the systemic corticosteroid doses should be increased temporarily and thereafter withdrawal should continue more slowly. During periods of stress or a severe asthma attack, transfer patients may require supplementary treatment with systemic corticosteroids.
Directions for Use
Illustrated Patient Information and Instructions for Use accompany each package of PULMICORT FLEXHALER.
Patients should be instructed to prime PULMICORT FLEXHALER prior to its initial use, and instructed to inhale deeply and forcefully each time the unit is used. Rinsing the mouth after inhalation is also recommended (see further instructions in PRECAUTIONS, Information for Patients).
HOW SUPPLIED
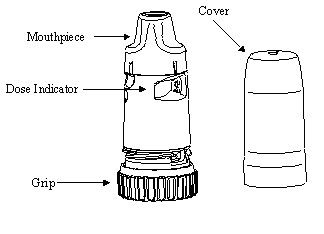
PULMICORT FLEXHALER consists of a number of assembled plastic details, the main parts being the dosing mechanism, the storage unit for drug substance, and the mouthpiece. The inhaler is protected by a white outer tubular cover screwed onto the inhaler. The body of the inhaler is white and the turning grip is brown. The PULMICORT FLEXHALER inhaler cannot be refilled and should be discarded when empty.
PULMICORT FLEXHALER is available in two strengths: 180 mcg/dose, 120 doses (NDC 0186-0916-12) with a target fill weight of 225 mg (range 200-250), and 90 mcg/dose, 60 doses (NDC 0186-0917-06) with a target fill weight of 165 mg (range 140-190).
The number in the middle of the dose indicator window shows how many doses are left in the inhaler. The inhaler is empty when the number zero (“0”) on the red background reaches the middle of the window. If the unit is used beyond the point at which the zero reaches the middle of the window, the correct amount of medication may not be obtained and the unit should be discarded.
Store in a dry place at controlled room temperature 20-25°C (68-77°F) [see USP] with the cover tightly in place. Keep out of the reach of children.
All trademarks are the property of the AstraZeneca group of companies
© AstraZeneca 2007, 2008
Manufactured for: AstraZeneca LP, Wilmington, DE 19850
By: AstraZeneca AB, Södertälje, Sweden
33023–05
Rev. 04/09
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


SUPPLEMENTAL PATIENT MATERIAL
Patient Information and Instructions for Use
PULMICORT FLEXHALER™ 180 mcg
(budesonide inhalation powder, 180 mcg)
PULMICORT FLEXHALER™ 90 mcg
(budesonide inhalation powder, 90 mcg)
(bew DEH so nide)
For Oral Inhalation Only
Rx Only
Please read this leaflet carefully before you start to take your medicine. It provides a summary of information on your medicine. Following these instructions helps to ensure that you are inhaling the medication correctly.
For further information ask your doctor, healthcare professional, or pharmacist.
What You Should Know about PULMICORT FLEXHALER
Your doctor or healthcare professional has prescribed PULMICORT FLEXHALER 180 mcg or PULMICORT FLEXHALER 90 mcg. Both contain a medication called budesonide, which is a synthetic corticosteroid. Corticosteroids are natural substances found in the body that help fight inflammation. They are used to treat asthma because they reduce the swelling and irritation in the walls of the small air passages in the lungs and ease breathing problems. When inhaled regularly, corticosteroids also help to prevent attacks of asthma.
PULMICORT FLEXHALER treats the inflammation—the “quiet part” of asthma that you cannot hear, see, or feel. When inflammation is left untreated, asthma symptoms and attacks can increase. PULMICORT FLEXHALER works to prevent and reduce your asthma symptoms and attacks.
Important Points to Remember about PULMICORT FLEXHALER
❶Make sure that this medicine is suitable for you (see “Before Using your PULMICORT FLEXHALER”).
❷It is important that you inhale each dose as your doctor or healthcare professional has advised.
❸Do not stop treatment or reduce your dose even if you feel better, unless told to do so by your doctor or healthcare professional. Use PULMICORT FLEXHALER as directed by your doctor or healthcare professional.
❹Do not inhale more doses or use your PULMICORT FLEXHALER more often than instructed by your doctor or healthcare professional.
❺You may not feel or taste the medication from your PULMICORT FLEXHALER when you inhale it. This does not mean that you did not get the medication. You should not repeat your inhalations even if you did not feel the medication when inhaling.
❻This medicine is not intended to provide rapid relief of your breathing difficulties during an asthma attack. It must be taken at regular intervals as recommended by your doctor or healthcare professional, and not as an emergency measure. Always have a short-acting bronchodilator medicine with you to treat sudden symptoms. If you do not have an inhaled, short-acting bronchodilator, contact your healthcare professional to have one prescribed for you.
❼Please contact your doctor or healthcare professional if:
· an asthma attack does not respond to the additional medication,
· you require more of the additional medication than usual.
❽ If you also use another medicine by inhalation, you should consult your doctor or healthcare professional for instructions on when to use it in relation to using your PULMICORT FLEXHALER.
What is PULMICORT FLEXHALER?
PULMICORT FLEXHALER is an inhaled corticosteroid approved for adults and children 6 years of age and older. It is a long-term controller (preventive or maintenance) asthma medicine. PULMICORT FLEXHALER does not treat the symptoms of a sudden asthma attack. Always have a short-acting bronchodilator medicine (rescue inhaler) with you. Inhaled corticosteroids help to decrease inflammation in the lungs. Inflammation in the lungs can lead to asthma symptoms.
What is Asthma?
Asthma is a condition that interferes with your breathing by preventing air from flowing freely into and out of the lungs. In patients with asthma, the airways become inflamed (swollen and irritated) and the muscles around the airways tighten.
How Does PULMICORT FLEXHALER Work?
PULMICORT FLEXHALER is a dry powder inhaler that delivers medication through inhalation as you deeply inhale. The inhaler delivers your medicine as a very fine powder. PULMICORT FLEXHALER helps reduce inflammation and helps keep the airways open to reduce asthma symptoms.
Before Using your PULMICORT FLEXHALER
Tell your doctor or healthcare professional before starting to take this medicine if you:
- are pregnant (or intending to become pregnant),
-
are breast-feeding a baby,
-
are allergic to any ingredient (see What are the Ingredients in PULMICORT FLEXHALER?). PULMICORT FLEXHALER contains small amounts of lactose (milk sugar), which contains trace levels of milk proteins. It is possible that cough, wheezing, or bronchospasm may occur in patients who have a severe milk protein allergy.
-
have any infections,
-
have or had tuberculosis,
-
have osteoporosis,
-
have recently been around anyone with chicken pox or measles,
-
are planning to have surgery,
-
have been taking an oral corticosteroid medicine like prednisone. You may have to follow specific instructions to avoid health risks associated with stopping the use of these types of medicines.
In some circumstances, this medicine may not be suitable and your doctor or healthcare professional may wish to prescribe a different medicine. Make sure that your doctor or healthcare professional knows what other medicines you are taking including prescription and non-prescription medicines, as well as any vitamins or dietary and herbal supplements.
What are the Possible Side Effects of PULMICORT FLEXHALER?
As with all inhaled corticosteroids, you should be aware of the following side effects:
-
Increased wheezing right after taking PULMICORT FLEXHALER. Always have a short-acting bronchodilator medicine with you to treat sudden wheezing. Short-acting bronchodilator medicines help to relax the muscles around the airways in your lungs. Wheezing happens when the muscles around the airways tighten. This makes it hard to breathe. In severe cases, wheezing can stop your breathing and cause death if not treated right away.
-
Immune system effects and a higher chance of infections.
-
Eye problems including glaucoma and cataracts. Eye examinations should be considered while using PULMICORT FLEXHALER.
-
A child’s growth should be checked regularly while taking PULMICORT FLEXHALER because of the potential for slowed growth.
Based on clinical trials, the most common side effects reported by patients using PULMICORT FLEXHALER are:
-
Sore nose and throat
-
Stuffy nose
-
Hay fever
-
Viral infections in the upper respiratory tract
These are not all of the possible side effects of PULMICORT FLEXHALER. For more information, ask your doctor, healthcare professional, or pharmacist. You may report side effects to FDA at 1-800-FDA-1088.
Using Your PULMICORT FLEXHALER
-
Follow the instructions shown in the section “How toUse Your PULMICORT FLEXHALER”. If you have any questions, talk with your doctor, healthcare professional or pharmacist.
-
It is important that you inhale each dose as directed by your doctor or healthcare professional. The pharmacy label will usually tell you what dose to take and how often. If it doesn’t, or if you are not sure, ask your doctor, healthcare professional, or pharmacist.
Dosage
-
You can determine which strength of PULMICORT FLEXHALER you have (either 180 mcg or 90 mcg) by looking at the label on the cover or by what is printed on the inhaler itself.
-
Use as directed by your doctor or healthcare professional.
-
It is very important that you follow your doctor or healthcare professional’s instructions as to how many inhalations to take and how often to use your PULMICORT FLEXHALER.
-
Do not inhale more doses or use your PULMICORT FLEXHALER more often than your doctor or healthcare professional advises.
-
You may not sense the presence of any medication entering your lungs when inhaling from PULMICORT FLEXHALER. This lack of sensation does not mean that you did not get the medication. You should not repeat your inhalations even if you did not feel the medication when inhaling.
-
It may take 1 to 2 weeks or longer before you feel maximum improvement, so it is very important that you use PULMICORT FLEXHALER regularly. Do not stop treatment or reduce your dose even if you are feeling better, unless told to do so by your doctor or healthcare professional.
-
If you miss a dose, just take your regularly scheduled next dose when it is due. Do not double the dose.
What are the Ingredients in PULMICORT FLEXHALER?
PULMICORT FLEXHALER contains budesonide as the active ingredient and lactose (which contains trace levels of milk proteins), an inactive ingredient (see Before Using your PULMICORT FLEXHALER).
How to Use Your PULMICORT FLEXHALER
Read the complete instructions carefully and use only as directed.
PRIMING INSTRUCTIONS:
Before you use a new PULMICORT FLEXHALER for the first time, you must prime it. To prime your PULMICORT FLEXHALER, follow the steps below:
1. Hold the unit so that the white cover points upward, grasp the inhaler by the brown grip in one hand and with the other hand, turn the white cover and lift it off.
2. Hold PULMICORT FLEXHALER by the brown grip, in the upright position (with mouthpiece up) in one hand. Using the thumb and index finger of your other hand, grasp the inhaler in the middle. Do not hold the inhaler at the top of the mouthpiece.
3. Twist the brown grip as far as it will go in one direction and then fully back again in the other direction until it stops (it does not matter which way you turn it first). You will hear a click during one of the twisting movements.
4. Repeat Step 3. Your PULMICORT FLEXHALER is now primed.
You do not have to prime it any other time after this, even if you put it aside for a prolonged period of time.
Now you are ready to load your first dose (see instructions for “loading a dose”).
TAKING A DOSE:
➊LOADING A DOSE
-
Twist the cover and lift it off.
-
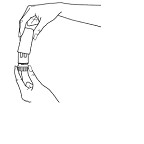
In order to load the correct dose, PULMICORT FLEXHALER must be held in the upright position (mouthpiece up) whenever a dose of medication is being loaded.
-
Do not hold the mouthpiece when you load the inhaler.
-
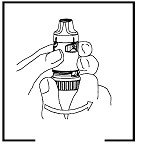
Twist the brown grip fully in one direction as far as it will go. Twist it fully back again in the other direction as far as it will go (it does not matter which way you turn it first).
Twist
-
You will hear a click during one of the twisting movements.
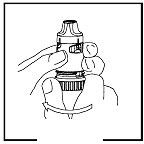
Click
-
PULMICORT FLEXHALER is designed to deliver only one dose at a time, no matter how often you click the brown grip, although the dose indicator will continue to advance. If you accidentally blow into your inhaler after loading a dose, simply follow the instructions for loading a new dose.
-
Do not shake the inhaler after loading it.
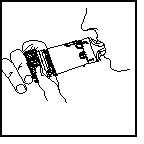
➋INHALING THE DOSE
Inhale
-
Turn your head away from the inhaler and breathe out.
-
Place the mouthpiece in your mouth, close your lips around the mouthpiece, and inhale deeply and forcefully through the inhaler.
-
You may not sense the presence of any medication entering your lungs when inhaling from PULMICORT FLEXHALER. This lack of sensation does not mean that you did not get the medication. You should not repeat your inhalations even if you did not feel the medication when inhaling.
-
Do not chew or bite on the mouthpiece.
-
Remove the inhaler from your mouth and exhale. Do not blow or exhale into the mouthpiece.
-
If more than one dose is required, just repeat the steps above.
-
When you are finished, place the cover back on the inhaler and twist shut. Rinse your mouth with water after each dose to reduce the risk of developing thrush. Do not swallow.
-
Do not use PULMICORT FLEXHALER if it has been damaged or if the mouthpiece has become detached.
Reading the Dose Indicator Window
-
The label on the box or cover will tell you how many doses are in your PULMICORT FLEXHALER.
-
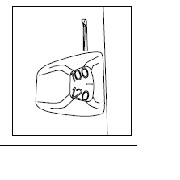
Your PULMICORT FLEXHALER has a convenient dose indicator window just below the mouthpiece. The dose indicator tells approximately how many doses remain in the inhaler. Look at the middle of the window to find out how many doses are left in your inhaler.
-
The dose indicator is connected to the turning grip and moves (counts down) every time a dose is loaded. It is unlikely that you will see the dose indicator move with each individual dose. Advancement of the indicator can be seen after intervals of 5 or so doses.
-
The dose indicator starts with either the number 60 or 120 when full, depending upon the strength of the product. The indicator is marked in intervals of 10 doses, alternating numbers and dashes, down to “0”.
|
60 Dose Inhaler |
120 Dose Inhaler | |
|
20 - 40 - 60 |
80 - 100 - 120 |
Dose indicator starts at 60 or 120 depending on strength (90 mcg or 180 mcg) of the product and counts down to 0. |
-
The dose indicator will always tell approximately how many doses are left in the PULMICORT FLEXHALER. The dose indicator counts down each time a dose is loaded, not when a dose is inhaled.
-
If you complete the instructions for loading the dose more than one time before you inhale the dose, you will only receive one dose. However, the dose indicator will advance slightly but it is unlikely that you will see the dose indicator move with each individual dose.
-
Your inhaler is empty when the number 0 on the red background has reached the middle of the window. At this time, your inhaler should be discarded, as it may no longer deliver the correct amount of medication, even though it may not feel completely empty and may seem like it continues to work..
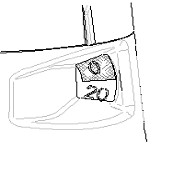
(You may still hear a sound if you shake it—this sound is not the medicine. This sound is produced by the drying agent inside.)
-
The grip will still twist and “click” even when your inhaler is empty.
-
Remember, you will get a new inhaler each time you refill your prescription.
-
Do not put your PULMICORT FLEXHALER in water (do not immerse it) to find out if it is empty. Simply check your dose indicator window.
Cleaning Your PULMICORT FLEXHALER
-
Keep your PULMICORT FLEXHALER clean and dry at all times. Do not immerse it in water.
-
Wipe the outside of the mouthpiece once a week with a dry tissue.
-
Do not use water or liquids when cleaning the mouthpiece.
-
Do not try to remove the mouthpiece or twist it unnecessarily.
Storing Your PULMICORT FLEXHALER
-
After each use, place the white cover back on and twist it tightly into place.
-
Store PULMICORT FLEXHALER in a dry place at controlled room temperature, 68-77°F (20-25°C) with the cover tightly in place.
-
Keep your PULMICORT FLEXHALER and all medicines in a secure place out of the reach of young children.
-
Do not use after the date shown on the body of your inhaler.
Further Information about PULMICORT FLEXHALER
-
PULMICORT FLEXHALER delivers your medicine as a very fine powder. Because of this, you may not feel or taste the medication from your PULMICORT FLEXHALER when you inhale it. This does not mean that you did not get the medication. Do not repeat your inhalations even if you did not feel the medication when inhaling.
-
PULMICORT FLEXHALER should not be used with a spacer.
This leaflet does not contain the complete information about your medicine. If you have any questions, or are not sure about something, then you should ask your doctor, healthcare professional, or pharmacist.
You may want to read this leaflet again. Please do not throw it away until you have finished your medicine.
Remember: This medicine has been prescribed for you by your doctor or healthcare professional. Do not give this medicine to anyone else.
Use this product as directed, unless instructed to do otherwise by your doctor or healthcare professional.
For additional information about PULMICORT FLEXHALER, please visit our website: pulmicortflexhaler.com or call the AstraZeneca Information Center at: 1-800-236-9933, Monday through Friday, 8 am – 7 pm ET, excluding holidays
PULMICORT FLEXHALER is a trademark of the AstraZeneca group of companies.
©AstraZeneca 2007, 2008
Manufactured for: AstraZeneca LP, Wilmington DE 19850 By: AstraZeneca AB, Södertälje, Sweden
Product of Sweden
| PULMICORT
FLEXHALER
budesonide aerosol, powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021949 | 10/06/2009 | |
| PULMICORT
FLEXHALER
budesonide aerosol, powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA021949 | 10/06/2009 | |
| Labeler - AstraZeneca LP (176500158) |
| Registrant - AstraZeneca PLC (230790719) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AstraZeneca AB | 353951254 | API MANUFACTURE, MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AstraZeneca LP | 176500158 | RELABEL, REPACK | |