DILTIAZEM HYDROCHLORIDE
-
diltiazem hydrochloride capsule, extended release
DILTIAZEM HYDROCHLORIDE
-
diltiazem hydrochloride capsule, extended release
TEVA Pharmaceuticals USA Inc.
----------
DILTIAZEM HYDROCHLORIDE Extended-release Capsules, USP(Once-a-Day Dosage)
Rx only
DESCRIPTION
Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-Benzothiazepin-4(5H)-one,3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride, (+)-cis-. The structural formula is:
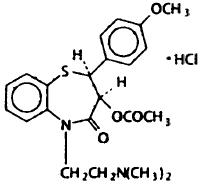
Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, methanol and chloroform. It has a molecular weight of 450.99. Diltiazem Hydrochloride Extended-release Capsule (Once-a-Day Dosage), for oral administration, is formulated as a once-a-day extended-release capsule containing either 120 mg, 180 mg, 240 mg, or 300 mg diltiazem hydrochloride.
Also contains: D&C Yellow #10, FD&C Green #3, Gelatin, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyacrylate Dispersion 30%, Polysorbate, Povidone, Simethicone, Sucrose Stearate, Talc, Titanium Dioxide.
Diltiazem Hydrochloride Extended-release Capsule meets USP Dissolution Test 12.
CLINICAL PHARMACOLOGY
The therapeutic effects of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) are believed to be related to its ability to inhibit the influx of calcium ions during membrane depolarization of cardiac and vascular smooth muscle.
Mechanisms of Action
Hypertension. Diltiazem produces its antihypertensive effect primarily by relaxation of vascular smooth muscle and the resultant decrease in peripheral vascular resistance. The magnitude of blood pressure reduction is related to the degree of hypertension; thus hypertensive individuals experience an antihypertensive effect, whereas there is only a modest fall in blood pressure in normotensives.
Angina. Diltiazem has been shown to produce increases in exercise tolerance, probably due to its ability to reduce myocardial oxygen demand. This is accomplished via reductions in heart rate and systemic blood pressure at submaximal and maximal work loads. Diltiazem has been shown to be a potent dilator of coronary arteries, both epicardial and subendocardial. Spontaneous and ergonovine-induced coronary artery spasm are inhibited by diltiazem.
In animal models, diltiazem interferes with the slow inward (depolarizing) current in excitable tissue. It causes excitation-contraction uncoupling in various myocardial tissues without changes in the configuration of the action potential. Diltiazem produces relaxation of coronary vascular smooth muscle and dilation of both large and small coronary arteries at drug levels which cause little or no negative inotropic effect. The resultant increases in coronary blood flow (epicardial and subendocardial) occur in ischemic and nonischemic models and are accompanied by dose-dependent decreases in systemic blood pressure and decreases in peripheral resistance.
Hemodynamic and Electrophysiologic Effects
Like other calcium channel antagonists, diltiazem decreases sinoatrial and atrioventricular conduction in isolated tissues and has a negative inotropic effect in isolated preparations. In the intact animal, prolongation of the AH interval can be seen at higher doses.
In man, diltiazem prevents spontaneous and ergonovine-provoked coronary artery spasm. It causes a decrease in peripheral vascular resistance and a modest fall in blood pressure in normotensive individuals, and in exercise tolerance studies in patients with ischemic heart disease, reduces the heart rate-blood pressure product for any given work load. Studies to date, primarily in patients with good ventricular function, have not revealed evidence of a negative inotropic effect; cardiac output, ejection fraction, and left ventricular end diastolic pressure have not been affected. Such data has no predictive value with respect to effects in patients with poor ventricular function, and increased heart failure has been reported in patients with preexisting impairment of ventricular function. There are as yet few data on the interaction of diltiazem and beta-blockers in patients with poor ventricular function. Resting heart rate is usually slightly reduced by diltiazem.
In hypertensive patients, diltiazem hydrochloride extended-release produces antihypertensive effects both in the supine and standing positions. In a double-blind, parallel, dose-response study utilizing doses ranging from 90 to 540 mg once daily, Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) lowered supine diastolic blood pressure in an apparent linear manner over the entire dosage range studied. The changes in diastolic blood pressure, measured at trough, for placebo, 90 mg, 180 mg, 360 mg and 540 mg were -2.9, -4.5, -6.1, -9.5, and -10.5 mm Hg, respectively. Postural hypotension is infrequently noted upon suddenly assuming an upright position. No reflex tachycardia is associated with the chronic antihypertensive effects. Diltiazem decreases vascular resistance, increases cardiac output (by increasing stroke volume), and produces a slight decrease or no change in heart rate. During dynamic exercise, increases in diastolic pressure are inhibited, while maximum achievable systolic pressure is usually reduced. Chronic therapy with diltiazem produces no change or an increase in plasma catecholamines. No increased activity of the renin-angiotensin-aldosterone axis has been observed. Diltiazem reduces the renal and peripheral effects of angiotensin II. Hypertensive animal models respond to diltiazem with reductions in blood pressure and increased urinary output and natriuresis without a change in urinary sodium/potassium ratio.
In a double-blind, parallel dose-response study of doses from 60 mg to 480 mg once daily, diltiazem hydrochloride extended-release capsules (once-a-day dosage) increased time to termination of exercise in a linear manner over the entire dose range studied. The improvement in time to termination of exercise utilizing a Bruce exercise protocol, measured at trough, for placebo, 60 mg, 120 mg, 240 mg, 360 mg, and 480 mg was 29, 40, 56, 51, 69 and 68 seconds, respectively. As doses of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) were increased, overall angina frequency was decreased. Diltiazem hydrochloride extended-release capsule (once-a-day dosage) 180 mg once daily, or placebo was administered in a double-blind study to patients receiving concomitant treatment with long-acting nitrates and/or beta-blockers. A significant increase in time to termination of exercise and a significant decrease in overall angina frequency was observed. In this trial the overall frequency of adverse events in the Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) treatment group was the same as the placebo group.
Intravenous diltiazem hydrochloride in doses of 20 mg prolongs AH conduction time and AV node functional and effective refractory periods by approximately 20%. In a study involving single oral doses of 300 mg of diltiazem hydrochloride in six normal volunteers, the average maximum PR prolongation was 14% with no instances of greater than first-degree AV block. Diltiazem associated prolongation of the AH interval is not more pronounced in patients with first-degree heart block. In patients with sick sinus syndrome, diltiazem significantly prolongs sinus cycle length (up to 50% in some cases).
Chronic oral administration of diltiazem hydrochloride to patients in doses of up to 540 mg/day has resulted in small increases in PR interval, and on occasion produces abnormal prolongation.(See WARNINGS).
Pharmacokinetics and Metabolism
Diltiazem is well absorbed from the gastrointestinal tract and is subject to an extensive first-pass effect, giving an absolute bioavailability (compared to intravenous administration) of about 40%. Diltiazem undergoes extensive metabolism in which only 2% to 4% of the unchanged drug appears in the urine. Drugs which induce or inhibit hepatic microsomal enzymes may alter diltiazem disposition.
Total radioactivity measurement following short IV administration in healthy volunteers suggests the presence of other unidentified metabolites, which attain higher concentrations than those of diltiazem and are more slowly eliminated; half-life of total radioactivity is about 20 hours compared to 2 to 5 hours for diltiazem.
In vitro binding studies show diltiazem is 70% to 80% bound to plasma proteins. Competitive in vitro ligand binding studies have also shown diltiazem hydrochloride binding is not altered by therapeutic concentrations of digoxin, hydrochlorothiazide, phenylbutazone, propranolol, salicylic acid, or warfarin. The plasma elimination half-life following single or multiple drug administration is approximately 3.0 to 4.5 hours. Desacetyl diltiazem is also present in the plasma at levels of 10% to 20% of the parent drug and is 25% to 50% as potent as a coronary vasodilator as diltiazem. Minimum therapeutic plasma diltiazem concentrations appear to be in the range of 50 to 200 ng/mL. There is a departure from linearity when dose strengths are increased; the half-life is slightly increased with dose. A study that compared patients with normal hepatic function to patients with cirrhosis found an increase in half-life and a 69% increase in bioavailability in the hepatically impaired patients. A single study in nine patients with severely impaired renal function showed no difference in the pharmacokinetic profile of diltiazem compared to patients with normal renal function.
Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage). When compared to a regimen of diltiazem hydrochloride tablets at steady-state, more than 95% of drug is absorbed from the diltiazem hydrochloride extended-release capsules formulation. A single 360-mg dose of the capsule results in detectable plasma levels within 2 hours and peak plasma levels between 10 and 14 hours; absorption occurs throughout the dosing interval. When Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) were coadministered with a high fat content breakfast, the extent of diltiazem absorption was not affected. Dose-dumping does not occur. The apparent elimination half-life after single or multiple dosing is 5 to 8 hours. A departure from linearity similar to that seen with diltiazem hydrochloride tablets and diltiazem hydrochloride extended-release capsules (twice-a-day dosage) is observed. As the dose of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) is increased from a daily dose of 120 mg to 240 mg, there is an increase in the area-under-the curve of 2.7 times. When the dose is increased from 240 mg to 360 mg, there is an increase in the area-under-the-curve of 1.6 times.
INDICATIONS AND USAGE
Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medications.
Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) are indicated for the management of chronic stable angina and angina due to coronary artery spasm.
CONTRAINDICATIONS
Diltiazem is contraindicated in (1) patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker, (2) patients with second- or third-degree AV block except in the presence of a functioning ventricular pacemaker, (3) patients with hypotension (less than 90 mm Hg systolic), (4) patients who have demonstrated hypersensitivity to the drug, and (5) patients with acute myocardial infarction and pulmonary congestion documented by x-ray on admission.
WARNINGS
1. Cardiac Conduction. Diltiazem prolongs AV node refractory periods without significantly prolonging sinus node recovery time, except in patients with sick sinus syndrome. This effect may rarely result in abnormally slow heart rates (particularly in patients with sick sinus syndrome) or second- or third-degree AV block (13 of 3290 patients or 0.40%). Concomitant use of diltiazem with beta-blockers or digitalis may result in additive effects on cardiac conduction. A patient with Prinzmetal's angina developed periods of asystole (2 to 5 seconds) after a single dose of 60 mg of diltiazem (see ADVERSE REACTIONS).
2. Congestive Heart Failure. Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, hemodynamic studies in humans with normal ventricular function have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). An acute study of oral diltiazem in patients with impaired ventricular function (ejection fraction 24% ± 6%) showed improvement in indices of ventricular function without significant decrease in contractile function (dp/dt). Worsening of congestive heart failure has been reported in patients with preexisting impairment of ventricular function. Experience with the use of diltiazem in combination with beta-blockers in patients with impaired ventricular function is limited. Caution should be exercised when using this combination.
3. Hypotension. Decreases in blood pressure associated with diltiazem therapy may occasionally result in symptomatic hypotension.
4. Acute Hepatic Injury. Mild elevations of transaminases with and without concomitant elevation in alkaline phosphatase and bilirubin have been observed in clinical studies. Such elevations were usually transient and frequently resolved even with continued diltiazem treatment. In rare instances, significant elevations in enzymes such as alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted. These reactions tended to occur early after therapy initiation (1 to 8 weeks) and have been reversible upon discontinuation of drug therapy. The relationship to diltiazem is uncertain in some cases, but probable in some. (See PRECAUTIONS).
PRECAUTIONS
General
Diltiazem hydrochloride is extensively metabolized by the liver and excreted by the kidneys and in bile. As with any drug given over prolonged periods, laboratory parameters of renal and hepatic function should be monitored at regular intervals. The drug should be used with caution in patients with impaired renal or hepatic function. In subacute and chronic dog and rat studies designed to produce toxicity, high doses of diltiazem were associated with hepatic damage. In special subacute hepatic studies, oral doses of 125 mg/kg and higher in rats were associated with histological changes in the liver, which were reversible when the drug was discontinued. In dogs, doses of 20 mg/kg were also associated with hepatic changes; however, these changes were reversible with continued dosing.
Dermatological events (see ADVERSE REACTIONS) may be transient and may disappear despite continued use of diltiazem. However, skin eruptions progressing to erythema multiforme and/or exfoliative dermatitis have also been infrequently reported. Should a dermatologic reaction persist, the drug should be discontinued.
Drug Interactions
Due to the potential for additive effects, caution and careful titration are warranted in patients receiving diltiazem concomitantly with other agents known to affect cardiac contractility and/or conduction (See WARNINGS). Pharmacologic studies indicate that there may be additive effects in prolonging AV conduction when using beta-blockers or digitalis concomitantly with diltiazem (See WARNINGS).
As with all drugs, care should be exercised when treating patients with multiple medications. Diltiazem is both a substrate and an inhibitor of the cytochrome P-450 3A4 enzyme system. Other drugs that are specific substrates, inhibitors, or inducers of this enzyme system may have a significant impact on the efficacy and side effect profile of diltiazem. Patients taking other drugs that are substrates of CYP450 3A4, especially in patients with renal and/or hepatic impairment, may require dosage adjustment when starting or stopping concomitantly administered diltiazem in order to maintain optimum therapeutic blood levels.
Anesthetics. The depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation associated with anesthetics may be potentiated by calcium channel blockers. When used concomitantly, anesthetics and calcium blockers should be titrated carefully.
Benzodiazepines. Studies showed that diltiazem increased the AUC of midazolam and triazolam by 3- to 4-fold and the Cmax by 2-fold, compared to placebo. The elimination half-life of midazolam and triazolam also increased (1.5- to 2.5-fold) during coadministration with diltiazem. These pharmacokinetic effects seen during diltiazem coadministration can result in increased clinical effects (e.g., prolonged sedation) of both midazolam and triazolam.
Beta-blockers. Controlled and uncontrolled domestic studies suggest that concomitant use of diltiazem and beta-blockers is usually well tolerated, but available data are not sufficient to predict the effects of concomitant treatment in patients with left ventricular dysfunction or cardiac conduction abnormalities.
Administration of diltiazem concomitantly with propranolol in five normal volunteers resulted in increased propranolol levels in all subjects and bioavailability of propranolol was increased approximately 50%. In vitro, propranolol appears to be displaced from its binding sites by diltiazem. If combination therapy is initiated or withdrawn in conjunction with propranolol, an adjustment in the propranolol dose may be warranted (see WARNINGS).
Buspirone. In nine healthy subjects, diltiazem significantly increased the mean buspirone AUC 5.5-fold and Cmax 4.1-fold compared to placebo. The T1/2 and Tmax of buspirone were not significantly affected by diltiazem. Enhanced effects and increased toxicity of buspirone may be possible during concomitant administration with diltiazem. Subsequent dose adjustments may be necessary during co-administration, and should be based on clinical assessment.
Carbamazepine. Concomitant administration of diltiazem with carbamazepine has been reported to result in elevated serum levels of carbamazepine (40% to 72% increase), resulting in toxicity in some cases. Patients receiving these drugs concurrently should be monitored for a potential drug interaction.
Cimetidine. A study in six healthy volunteers has shown a significant increase in peak diltiazem plasma levels (58%) and area-under-the-curve (53%) after a 1-week course of cimetidine at 1200 mg per day and a single dose of diltiazem 60 mg. Ranitidine produced smaller, nonsignificant increases. The effect may be mediated by cimetidine's known inhibition of hepatic cytochrome P-450, the enzyme system responsible for the first-pass metabolism of diltiazem. Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with cimetidine. An adjustment in the diltiazem dose may be warranted.
Cyclosporine. A pharmacokinetic interaction between diltiazem and cyclosporine has been observed during studies involving renal and cardiac transplant patients. In renal and cardiac transplant recipients, a reduction of cyclosporine dose ranging from 15% to 48% was necessary to maintain cyclosporine trough concentrations similar to those seen prior to the addition of diltiazem. If these agents are to be administered concurrently, cyclosporine concentrations should be monitored, especially when diltiazem therapy is initiated, adjusted, or discontinued. The effect of cyclosporine on diltiazem plasma concentrations has not been evaluated.
Digitalis. Administration of diltiazem with digoxin in 24 healthy male subjects increased plasma digoxin concentrations approximately 20%. Another investigator found no increase in digoxin levels in 12 patients with coronary artery disease. Since there have been conflicting results regarding the effect of digoxin levels, it is recommended that digoxin levels be monitored when initiating, adjusting, and discontinuing diltiazem therapy to avoid possible over- or under-digitalization (see WARNINGS).
Lovastatin. In a ten-subject study, coadministration of diltiazem (120 mg bid, diltiazem SR) with lovastatin resulted in 3- to 4-fold increase in mean lovastatin AUC and Cmax versus lovastatin alone; no change in pravastatin AUC and Cmax was observed during diltiazem coadministration. Diltiazem plasma levels were not significantly affected by lovastatin or pravastatin.
Quinidine. Diltiazem significantly increases the AUC(0 → ∞) of quinidine by 51%, T1/2 by 36%, and decreases its CLoral by 33%. Monitoring for quinidine adverse effects may be warranted and the dose adjusted accordingly.
Rifampin. Coadministration of rifampin with diltiazem lowered the diltiazem plasma concentrations to undetectable levels. Coadministration of diltiazem with rifampin or any known CYP3A4 inducer should be avoided when possible, and alternative therapy considered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats at oral dosage levels of up to 100 mg/kg/day, and a 21-month study in mice at oral dosage levels of up to 30 mg/kg/day showed no evidence of carcinogenicity. There was also no mutagenic response in vitro or in vivo in mammalian cell assays or in vitro in bacteria. No evidence of impaired fertility was observed in a study performed in male and female rats at oral dosages of up to 100 mg/kg/day.
Pregnancy
Category C. Reproduction studies have been conducted in mice, rats, and rabbits. Administration of doses ranging from five to ten times greater (on a mg/kg basis) than the daily recommended therapeutic dose has resulted in embryo and fetal lethality. These doses, in some studies, have been reported to cause skeletal abnormalities. In the perinatal/postnatal studies, there was an increased incidence of stillbirths at doses of 20 times the human dose or greater.
There are no well-controlled studies in pregnant women; therefore, use diltiazem in pregnant women only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Diltiazem is excreted in human milk. One report suggests that concentrations in breast milk may approximate serum levels. If use of diltiazem is deemed essential, an alternative method of infant feeding should be instituted.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of diltiazem did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Serious adverse reactions have been rare in studies carried out to date, but it should be recognized that patients with impaired ventricular function and cardiac conduction abnormalities have usually been excluded from these studies.
The following table presents the most common adverse reactions reported in placebo-controlled angina and hypertension trials in patients receiving Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) product up to 360 mg with rates in placebo patients shown for comparison.
| diltiazem hydrochloride extended-release capsules (once-a-day) Placebo-Controlled Angina and Hypertension Trials Combined |
||
|---|---|---|
| Adverse Reactions | diltiazem hydrochloride extended-release capsules (once-a-day) (n=607) | Placebo (once-a-day) (n=301) |
| Headache | 5.4% | 5.0% |
| Dizziness | 3.0% | 3.0% |
| Bradycardia | 3.3% | 1.3% |
| AV Block First Degree | 3.3% | 0.0% |
| Edema | 2.6% | 1.3% |
| ECG Abnormality | 1.6% | 2.3% |
| Asthenia | 1.8% | 1.7% |
In clinical trials of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage), diltiazem hydrochloride tablets, and diltiazem hydrochloride extended-release capsules involving over 3200 patients, the most common events (i.e. greater than 1%) were edema (4.6%), headache (4.6%), dizziness (3.5%), asthenia (2.6%), first-degree AV block (2.4%), bradycardia (1.7%), flushing (1.4%), nausea (1.4%) and rash (1.2%).
In addition, the following events were reported infrequently (less than 1%) in angina or hypertension trials:
Cardiovascular: Angina, arrhythmia, AV block (second- or third-degree), bundle branch block, congestive heart failure, ECG abnormalities, hypotension, palpitations, syncope, tachycardia, ventricular extrasystoles.
Nervous System: Abnormal dreams, amnesia, depression, gait abnormality, hallucinations, insomnia, nervousness, paresthesia, personality change, somnolence, tinnitus, tremor.
Gastrointestinal: Anorexia, constipation, diarrhea, dry mouth, dysgeusia, dyspepsia, mild elevations of SGOT, SGPT, LDH, and alkaline phosphatase (see WARNINGS, Acute Hepatic Injury), thirst, vomiting, weight increase.
Dermatological: Petechiae, photosensitivity, pruritus, urticaria.
Other: Amblyopia, CPK increase, dyspnea, epistaxis, eye irritation, hyperglycemia, hyperuricemia, impotence, muscle cramps, nasal congestion, nocturia, osteoarticular pain, polyuria, sexual difficulties.
The following postmarketing events have been reported infrequently in patients receiving diltiazem: allergic reactions, alopecia, angioedema (including facial or periorbital edema), asystole, erythema multiforme (including Stevens-Johnson syndrome, toxic epidermal necrolysis), exfoliative dermatitis, extrapyramidal symptoms, gingival hyperplasia, hemolytic anemia, increased bleeding time, leukopenia, purpura, retinopathy, myopathy, and thrombocytopenia. In addition, events such as myocardial infarction have been observed which are not readily distinguishable from the natural history of the disease in these patients. A number of well-documented cases of generalized rash, some characterized as leukocytoclastic vasculitis, have been reported. However, a definitive cause and effect relationship between these events and diltiazem therapy is yet to be established.
OVERDOSAGE
The oral LD50's in mice and rats range from 415 to 740 mg/kg and from 560 to 810 mg/kg, respectively. The intravenous LD50's in these species were 60 and 38 mg/kg, respectively. The oral LD50 in dogs is considered to be in excess of 50 mg/kg, while lethality was seen in monkeys at 360 mg/kg.
The toxic dose in man is not known. Due to extensive metabolism, blood levels after a standard dose of diltiazem can vary over tenfold, limiting the usefulness of blood levels in overdose cases.
There have been reports of diltiazem overdose in amounts ranging from <1 g to 18 g. Of cases with known outcome, most patients recovered and in cases with a fatal outcome, the majority involved multiple drug ingestions.
Events observed following diltiazem overdose included bradycardia, hypotension, heart block, and cardiac failure. Most reports of overdose described some supportive medical measure and/or drug treatment. Bradycardia frequently responded favorably to atropine as did heart block, although cardiac pacing was also frequently utilized to treat heart block. Fluids and vasopressors were used to maintain blood pressure, and in cases of cardiac failure, inotropic agents were administered. In addition, some patients received treatment with ventilatory support, gastric lavage, activated charcoal, and/or intravenous calcium.
The effectiveness of intravenous calcium administration to reverse the pharmacological effects of diltiazem overdose has been inconsistent. In a few reported cases, overdose with calcium channel blockers associated with hypotension and bradycardia that was initially refractory to atropine became more responsive to atropine after the patients received intravenous calcium. In some cases intravenous calcium has been administered (1 g calcium chloride or 3 g calcium gluconate) over 5 minutes, and repeated every 10-20 minutes as necessary. Calcium gluconate has also been administered as a continuous infusion at a rate of 2 g per hour for 10 hours. Infusions of calcium for 24 hours or more may be required. Patients should be monitored for signs of hypercalcemia.
In the event of overdose or exaggerated response, appropriate supportive measures should be employed in addition to gastrointestinal decontamination. Diltiazem does not appear to be removed by peritoneal or hemodialysis. Limited data suggest that plasmapheresis or charcoal hemoperfusion may hasten diltiazem eliminiation following overdose. Based on the known pharmacological effects of diltiazem and/or reported clinical experiences, the following measures may be considered:
Bradycardia: Administer atropine (0.60 to 1 mg). If there is no response to vagal blockage, administer isoproterenol cautiously.
High-degree AV Block: Treat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
Cardiac Failure: Administer inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics.
Hypotension: Vasopressors (e.g. dopamine or norepinephrine).
Actual treatment and dosage should depend on the severity of the clinical situation and the judgment and experience of the treating physician.
DOSAGE AND ADMINISTRATION
Patients controlled on diltiazem alone or in combination with other medications may be switched to Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) at the nearest equivalent total daily dose. Higher doses of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) may be needed in some patients. Patients should be closely monitored. Subsequent titration to higher or lower doses may be necessary and should be initiated as clinically warranted. There is limited general clinical experience with doses above 360 mg, but doses to 540 mg have been studied in clinical trials. The incidence of side effects increases as the dose increases with first-degree AV block, dizziness, and sinus bradycardia bearing the strongest relationship to dose.
Hypertension: Dosage needs to be adjusted by titration to individual patient needs. When used as monotherapy, reasonable starting doses are 180 to 240 mg once daily, although some patients may respond to lower doses. Maximum antihypertensive effect is usually observed by 14 days of chronic therapy; therefore, dosage adjustments should be scheduled accordingly. The usual dosage range studied in clinical trials was 240 to 360 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily.
Angina: Dosages for the treatment of angina should be adjusted to each patient's needs, starting with a dose of 120 or 180 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily. When necessary, titration may be carried out over a 7- to 14-day period.
Concomitant Use With Other Cardiovascular Agents
1. Sublingual NTG. May be taken as required to abort acute anginal attacks during Diltiazem Hydrochloride Extended-release (One-a-Day Dosage) therapy.
2. Prophylactic Nitrate Therapy. Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) may be safely coadministered with short-and long-acting nitrates.
3. Beta-blockers. (see WARNINGS and PRECAUTIONS.)
4. Antihypertensives. Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) have an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of Diltiazem Hydrochloride Extended-release Capsules (Once-a-Day Dosage) or the concomitant antihypertensives may need to be adjusted when adding one to the other.
HOW SUPPLIED
Diltiazem Hydrochloride Extended-release Capsules,USP
| Strength | Description | Qty. | NDC# |
|---|---|---|---|
| 120 mg | Light green | 90 btl | 0093-5112-98 |
| opaque capsule | 500 btl | 0093-5112-05 | |
| imprinted with BVF 120 | |||
| 180 mg | Dark green | 90 btl | 0093-5117-98 |
| opaque/light | 500 btl | 0093-5117-05 | |
| green opaque | |||
| capsule imprinted with BVF 180 | |||
| 240 mg | Dark green | 90 btl | 0093-5118-98 |
| opaque capsule | 500 btl | 0093-5118-05 | |
| imprinted with BVF 240 | |||
| 300 mg | Ivory | 90 btl | 0093-5119-98 |
| opaque/dark | 500 btl | 0093-5119-05 | |
| green opaque capsule imprinted with BVF 300 |
Storage Conditions:
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Avoid excessive humidity.
Dispense in tight, light-resistant container as defined in USP.
Manufactured by:
Biovail Corporation
Mississauga, ON
L5N 8M5, Canada
For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960, USA
Made in the U.S.A.
LB0004-02 Rev. 09/08
PRINCIPAL DISPLAY PANEL - 120 mg Capsules
NDC 0093-5112-98
DILTIAZEM
HYDROCHLORIDE
Extended-release
Capsules USP*
120 mg
Once-A-Day Dosage
Rx only
90 CAPSULES
TEVA

PRINCIPAL DISPLAY PANEL - 180 mg Capsules
NDC 0093-5117-98
DILTIAZEM
HYDROCHLORIDE
Extended-release
Capsules USP*
180 mg
Once-A-Day Dosage
Rx only
90 CAPSULES
TEVA

PRINCIPAL DISPLAY PANEL - 240 mg Capsules
NDC 0093-5118-98
DILTIAZEM
HYDROCHLORIDE
Extended-release
Capsules USP*
240 mg
Once-A-Day Dosage
Rx only
90 CAPSULES
TEVA

PRINCIPAL DISPLAY PANEL - 300 mg Capsules
NDC 0093-5119-98
DILTIAZEM
HYDROCHLORIDE
Extended-release
Capsules USP*
300 mg
Once-A-Day Dosage
Rx only
90 CAPSULES
TEVA

| DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA075116 | 08/19/2009 | |
| DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA075116 | 08/19/2009 | |
| DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA075116 | 08/19/2009 | |
| DILTIAZEM HYDROCHLORIDE
diltiazem hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA075116 | 08/19/2009 | |
| Labeler - TEVA Pharmaceuticals USA Inc. (118234421) |
| Registrant - Biovail Technologies LTD (830950668) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Biovail Corporation | 253292734 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Biovail Laboratories International SRL | 073701869 | MANUFACTURE | |