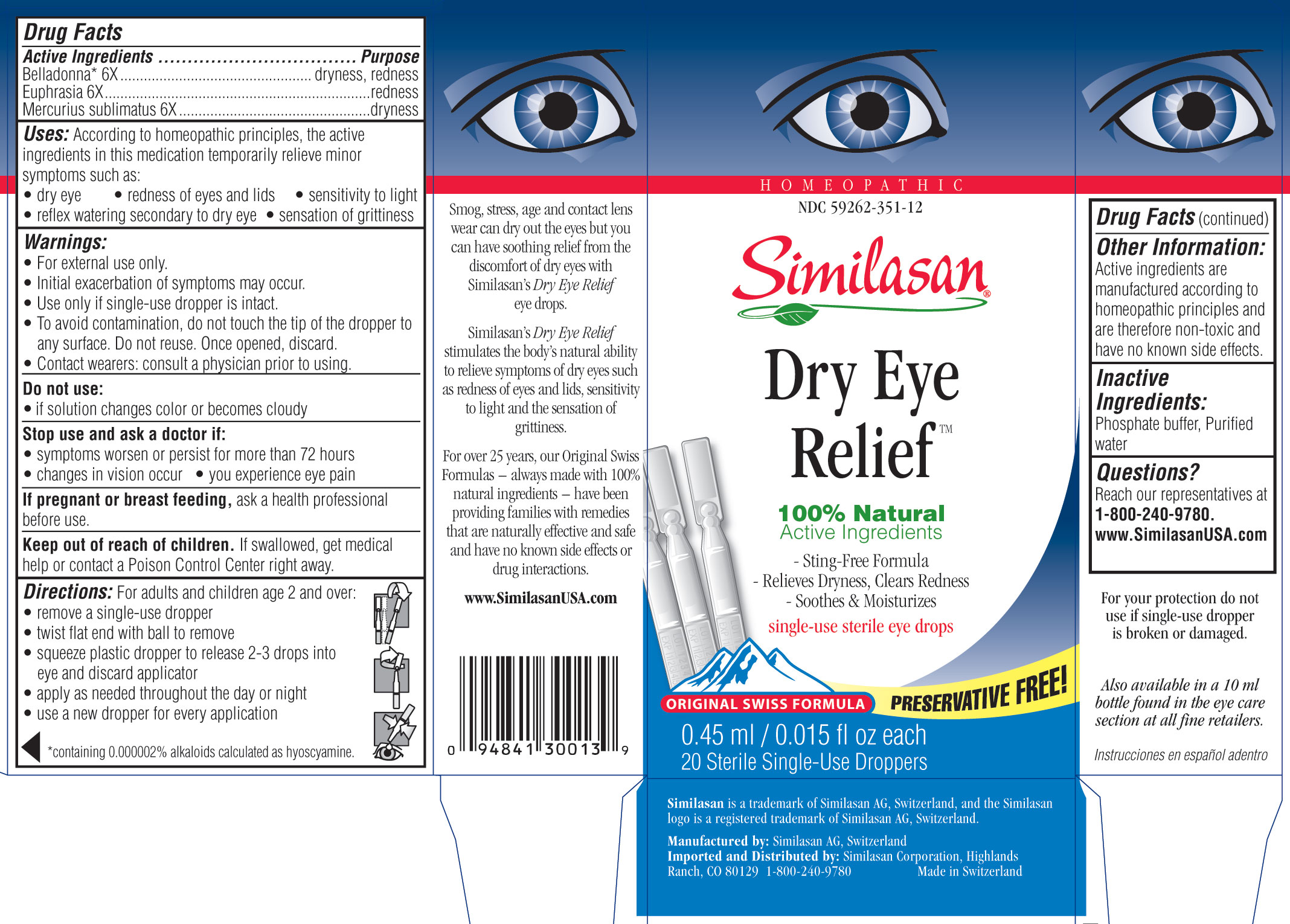
DRY EYE RELIEF- atropa belladonna, euphrasia stricta and mercuric chloride solution/ drops
Similasan AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Uses
According to homeopathic principles, the active ingredients in this medication temporarily minor symptoms such as:
- dry eye
- redness of eyes and lids
- reflex watering secondary to dry eye
- sensation of grittiness
- sensitivity to light
Warnings
- For external use only.
- Initial exacerbation of symptoms may occur.
- Use only if single-use dropper is intact.
- To avoid contamination, do not touch the tip of the dropper to any surface. Do not reuse. Once opened, discard.
- Contact wearers: consult a physician prior to using.
Directions
For adults and children age 2 and over:
- remove a single use dropper
- twist flat end with ball to remove
- squeeze plastic tip to release 2-3 drops into eye and discard applicator
- apply as needed throughout the day or night
- use a dropper for every application
Other information
Active ingredients are manufactured according to homeopathic principles and are therefore non-toxic and have no known side effects.
| DRY EYE RELIEF
belladonna 6x, euphrasia 6x, mercurius sublimatus 6x solution/ drops |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Similasan AG (481545754) |