ERYTHRA-DERM
-
erythromycin solution
Paddock Laboratories, Inc.
----------
Erythra-Derm™
Erythromycin Topical
Solution USP, 2%
DESCRIPTION:
Erythromycin is an antibiotic produced from a strain of Streptomyces erythraeus. It is basic and readily forms salts with acids.
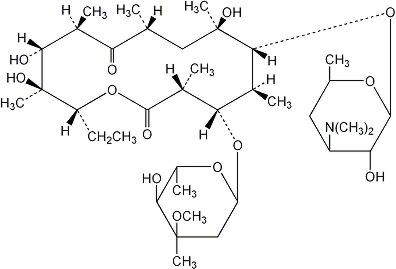
Chemically, erythromycin is (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-0-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2, 10-dione with a molecular formula of C37H67NO13 and a molecular weight of 733.94. The structural formula is as follows:
Erythromycin Topical Solution USP 2% contains 20 mg/mL of erythromycin base in a vehicle consisting of alcohol (66%), propylene glycol, and citric acid to adjust pH.
CLINICAL PHARMACOLOGY:
Although the mechanism by which Erythromycin Topical Solution acts in reducing inflammatory lesions of acne vulgaris is unknown, it is presumably due to its antibiotic action.
INDICATIONS AND USAGE:
Erythromycin Topical Solution is indicated for the topical control of acne vulgaris.
CONTRAINDICATIONS:
Erythromycin Topical Solution is contraindicated in persons who have shown hypersensitivity to erythromycin or any of the other listed ingredients.
WARNINGS:
The safe use of Erythromycin Topical Solution during pregnancy or lactation has not been established.
PRECAUTIONS:
General:
The use of antibiotic agents may be associated with the overgrowth of antibiotic-resistant organisms. If this occurs, administration of this drug should be discontinued and appropriate measures taken.
Information for Patients:
Erythromycin Topical Solution is for external use only and should be kept away from the eyes, nose, mouth and other mucous membranes. Concomitant topical acne therapy should be used with caution because a cumulative irritant effect may occur, especially with the use of peeling, desquamating or abrasive agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long term animal studies to evaluate carcinogenic potential, mutagenicity, or the effect on fertility of erythromycin have not been conducted.
Pregnancy:
Pregnancy Category C. Animal reproduction studies have not been conducted with erythromycin. It is also not known whether erythromycin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Erythromycin should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Erythromycin is excreted in breast milk. Caution must be exercised when erythromycin is administered to a nursing woman.
ADVERSE REACTIONS:
Adverse conditions reported with the use of erythromycin topical solutions include dryness, tenderness, pruritis, desquamation, erythema, oiliness, and burning sensation. Irritation of the eyes has also been reported. A case of generalized urticarial reaction, possibly related to the drug, which required the use of systemic steroid therapy has been reported.
DOSAGE AND ADMINISTRATION:
Erythromycin Topical Solution should be applied to the affected areas twice a day after the skin has been thoroughly washed with warm water and soap and patted dry. Apply contents after installing applicator (below) by tilting bottle and rubbing applicator lightly over affected areas until skin is thoroughly wetted. Close tightly after each use. Acne lesions on the face, neck, shoulder, chest, and back may be treated in this manner.
HOW SUPPLIED:
60 mL Bottle: NDC 0574-0014-02
Storage:
Keep tightly closed. Store at controlled room temperature, 15° - 30°C (59° - 86°F).
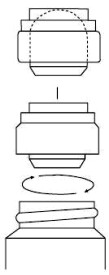
Instructions for installing applicator:
- Remove and discard shipping cap.
- Push applicator firmly into bottle using white cap as holder.
- Screw white cap down to seat applicator.
Paddock Laboratories, Inc.
Minneapolis, Minnesota 55427
(06-98)
| ERYTHRA-DERM
erythromycin solution |
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|||||||||||||||||||
Revised: 04/2009Paddock Laboratories, Inc.