REBIF - interferon beta-1a
REBIF REBIDOSE - interferon beta-1a
REBIF- interferon beta-1a injection, solution
REBIF - interferon beta-1a injection, solution
REBIF REBIDOSE- interferon beta-1a injection, solution
EMD Serono, Inc.
----------
DESCRIPTION
Rebif® (interferon beta-1a) is a purified 166 amino acid glycoprotein with a molecular weight of approximately 22,500 daltons. It is produced by recombinant DNA technology using genetically engineered Chinese Hamster Ovary cells into which the human interferon beta gene has been introduced. The amino acid sequence of Rebif® is identical to that of natural fibroblast derived human interferon beta. Natural interferon beta and interferon beta-1a (Rebif®) are glycosylated with each containing a single N-linked complex carbohydrate moiety.
Using a reference standard calibrated against the World Health Organization natural interferon beta standard (Second International Standard for Interferon, Human Fibroblast GB 23 902 531), Rebif® has a specific activity of approximately 270 million international units (MIU) of antiviral activity per mg of interferon beta-1a determined specifically by an in vitro cytopathic effect bioassay using WISH cells and Vesicular Stomatitis virus. Rebif® 8.8 mcg, 22 mcg and 44 mcg contains approximately 2.4 million international units , 6 million international units or 12 million international units, respectively, of antiviral activity using this method.
Rebif® (interferon beta-1a) is formulated as a sterile solution in a pre-filled syringe or Rebif® Rebidose® autoinjector intended for subcutaneous (sc) injection. Each 0.5 mL (0.5 cc) of Rebif® contain either 22 mcg or 44 mcg of interferon beta-1a, 2 mg or 4 mg albumin (human) USP, 27.3 mg mannitol USP, 0.4 mg sodium acetate, Water for Injection USP. Each 0.2 mL (0.2 cc) of Rebif® contains 8.8 mcg of interferon beta-1a, 0.8 mg albumin (human) USP, 10.9 mg mannitol USP, 0.16 mg sodium acetate, and Water for Injection USP.
CLINICAL PHARMACOLOGY
General
Interferons are a family of naturally occurring proteins that are produced by eukaryotic cells in response to viral infection and other biological inducers. Interferons possess immunomodulatory, antiviral and antiproliferative biological activities. They exert their biological effects by binding to specific receptors on the surface of cells. Three major groups of interferons have been distinguished: alpha, beta, and gamma. Interferons alpha and beta form the Type I interferons and interferon gamma is a Type II interferon. Type I interferons have considerably overlapping but also distinct biological activities. Interferon beta is produced naturally by various cell types including fibroblasts and macrophages. Binding of interferon beta to its receptors initiates a complex cascade of intracellular events that leads to the expression of numerous interferon-induced gene products and markers, including 2', 5'-oligoadenylate synthetase, beta 2-microglobulin and neopterin, which may mediate some of the biological activities. The specific interferon-induced proteins and mechanisms by which interferon beta-1a exerts its effects in multiple sclerosis have not been fully defined.
Pharmacokinetics
The pharmacokinetics of Rebif® (interferon beta-1a) in people with multiple sclerosis have not been evaluated. In healthy volunteer subjects, a single subcutaneous (sc) injection of 60 mcg of Rebif® (liquid formulation) resulted in a peak serum concentration (Cmax) of 5.1 ± 1.7 IU/mL (mean ± SD), with a median time of peak serum concentration (Tmax) of 16 hours. The serum elimination half-life (t1/2) was 69 ± 37 hours, and the area under the serum concentration versus time curve (AUC) from zero to 96 hours was 294 ± 81 IU h/mL. Following every other day sc injections in healthy volunteer subjects, an increase in AUC of approximately 240% was observed, suggesting that accumulation of interferon beta-1a occurs after repeat administration. Total clearance is approximately 33-55 L/hour. There have been no observed gender-related effects on pharmacokinetic parameters. Pharmacokinetics of Rebif® in pediatric and geriatric patients or patients with renal or hepatic insufficiency have not been established.
Pharmacodynamics
Biological response markers (e.g., 2', 5'-OAS activity, neopterin and beta 2-microglobulin) are induced by interferon beta-1a following parenteral doses administered to healthy volunteer subjects and to patients with multiple sclerosis. Following a single sc administration of 60 mcg of Rebif® intracellular 2', 5'-OAS activity peaked between 12 to 24 hours and beta-2-microglobulin and neopterin serum concentrations showed a maximum at approximately 24 to 48 hours. All three markers remained elevated for up to four days. Administration of Rebif® 22 mcg three times per week (tiw) inhibited mitogen-induced release of pro-inflammatory cytokines (IFN-γ, IL-1, IL-6, TNF-α and TNF-β) by peripheral blood mononuclear cells that, on average, was near double that observed with Rebif® administered once per week (qw) at either 22 or 66 mcg.
The relationships between serum interferon beta-1a levels and measurable pharmacodynamic activities to the mechanism(s) by which Rebif® exerts its effects in multiple sclerosis are unknown. No gender-related effects on pharmacodynamic parameters have been observed.
CLINICAL STUDIES
Two multicenter studies evaluated the safety and efficacy of Rebif® in patients with relapsing-remitting multiple sclerosis.
Study 1 was a randomized, double-blind, placebo controlled study in patients with multiple sclerosis for at least one year, Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 0 to 5, and at least 2 acute exacerbations in the previous 2 years.(1) Patients with secondary progressive multiple sclerosis were excluded from the study. Patients received sc injections of either placebo (n = 187), Rebif® 22 mcg (n = 189), or Rebif® 44 mcg (n = 184) administered three times per week for two years. Doses of study agents were progressively increased to their target doses during the first 4 to 8 weeks for each patient in the study (see DOSAGE AND ADMINISTRATION).
The primary efficacy endpoint was the number of clinical exacerbations. Numerous secondary efficacy endpoints were also evaluated and included exacerbation-related parameters, effects of treatment on progression of disability and magnetic resonance imaging (MRI)-related parameters. Progression of disability was defined as an increase in the EDSS score of at least 1 point sustained for at least 3 months. Neurological examinations were completed every 3 months, during suspected exacerbations, and coincident with MRI scans. All patients underwent proton density T2-weighted (PD/T2) MRI scans at baseline and every 6 months. A subset of 198 patients underwent PD/T2 and T1-weighted gadolinium-enhanced (Gd)-MRI scans monthly for the first 9 months. Of the 560 patients enrolled, 533 (95%) provided 2 years of data and 502 (90%) received 2 years of study agent.
Study results are shown in Table 1 and Figure 1. Rebif® at doses of 22 mcg and 44 mcg administered sc three times per week significantly reduced the number of exacerbations per patient as compared to placebo. Differences between the 22 mcg and 44 mcg groups were not significant (p >0.05).
The exact relationship between MRI findings and the clinical status of patients is unknown. Changes in lesion area often do not correlate with changes in disability progression. The prognostic significance of the MRI findings in these studies has not been evaluated.
| Placebo | 22 mcg tiw | 44 mcg tiw | |
|---|---|---|---|
| n = 187 | n = 189 | n = 184 | |
|
|||
| Exacerbation-related | |||
| Mean number of exacerbations per patient over 2 years*,† | 2.56 | 1.82‡ | 1.73§ |
| (Percent reduction) | (29%) | (32%) | |
| Percent (%) of patients exacerbation-free at 2 years¶ | 15% | 25%# | 32%§ |
| Median time to first exacerbation (months)*,Þ | 4.5 | 7.6‡ | 9.6§ |
| MRI | n = 172 | n = 171 | n = 171 |
| Median percent (%) change of MRI PD-T2 lesion area at 2 yearsß | 11.0% | -1.2%§ | -3.8%§ |
| Median number of active lesions per patient per scan (PD/T2; 6 monthly)ß | 2.25 | 0.75§ | 0.5§ |
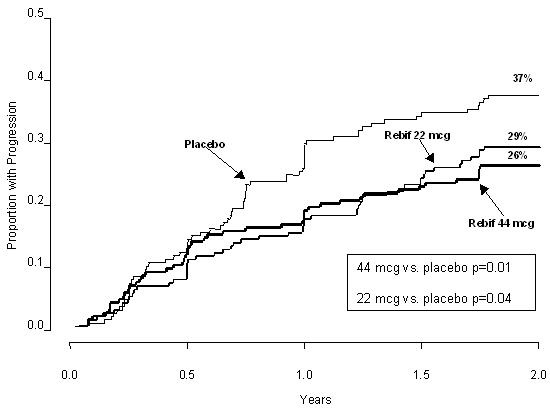
The time to onset of progression in disability sustained for three months was significantly longer in patients treated with Rebif® than in placebo-treated patients. The Kaplan-Meier estimates of the proportions of patients with sustained disability are depicted in Figure 1.
Figure 1: Proportions of Patients with Sustained Disability Progression
The safety and efficacy of treatment with Rebif® beyond 2 years have not been established.
Study 2 was a randomized, open-label, evaluator-blinded, active comparator study.(2) Patients with relapsing-remitting multiple sclerosis with EDSS scores ranging from 0 to 5.5, and at least 2 exacerbations in the previous 2 years were eligible for inclusion. Patients with secondary progressive multiple sclerosis were excluded from the study. Patients were randomized to treatment with Rebif® 44 mcg tiw by sc injection (n=339) or Avonex® 30 mcg qw by intramuscular (im) injection (n=338). Study duration was 48 weeks.
The primary efficacy endpoint was the proportion of patients who remained exacerbation-free at 24 weeks. The principal secondary endpoint was the mean number per patient per scan of combined unique active MRI lesions through 24 weeks, defined as any lesion that was T1 active or T2 active. Neurological examinations were performed every three months by a neurologist blinded to treatment assignment. Patient visits were conducted monthly, and mid-month telephone contacts were made to inquire about potential exacerbations. If an exacerbation was suspected, the patient was evaluated with a neurological examination. MRI scans were performed monthly and analyzed in a treatment–blinded manner.
Patients treated with Rebif® 44 mcg sc three times per week were more likely to remain relapse-free at 24 and 48 weeks than were patients treated with Avonex® 30 mcg im qw (Table 2). This study does not support any conclusion regarding effects on the accumulation of physical disability.
| Rebif® | Avonex® | Absolute Difference | Risk of relapse on Rebif® relative to Avonex® | |
|---|---|---|---|---|
| Relapses | N=339 | N=338 | ||
| Proportion of patients relapse-free at 24 weeks* | 75%† | 63% | 12% | 0.68 |
| (95% CI: 5%, 19%) | (95% CI: 0.54, 0.86) | |||
| Proportion of patients relapse-free at 48 weeks | 62%‡ | 52% | 10% | 0.81 |
| (95% CI: 2%, 17%) | (95% CI: 0.68, 0.96) | |||
| MRI (through 24 weeks) | N=325 | N=325 | ||
| Median of the mean number of combined unique MRI lesions per patient per scan§ (25th, 75th percentiles) | 0.17† | 0.33 | ||
| (0.00, 0.67) | (0.00, 1.25) | |||
The adverse reactions over 48 weeks were generally similar between the two treatment groups. Exceptions included injection site disorders (83% of patients on Rebif® vs. 28% of patients on Avonex®), hepatic function disorders (18% on Rebif® vs. 10% on Avonex®), and leukopenia (6% on Rebif® vs. <1% on Avonex®), which were observed with greater frequency in the Rebif® group compared to the Avonex® group.
INDICATIONS AND USAGE
Rebif® (interferon beta-1a) is indicated for the treatment of patients with relapsing forms of multiple sclerosis to decrease the frequency of clinical exacerbations and delay the accumulation of physical disability. Efficacy of Rebif® in chronic progressive multiple sclerosis has not been established.
CONTRAINDICATIONS
Rebif® (interferon beta-1a) is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon, human albumin, or any other component of the formulation.
WARNINGS
Depression and Suicide
Rebif® (interferon beta-1a) should be used with caution in patients with depression, a condition that is common in people with multiple sclerosis. Depression, suicidal ideation, and suicide attempts have been reported to occur with increased frequency in patients receiving interferon compounds, including Rebif®. In addition, there have been postmarketing reports of suicide in patients treated with Rebif®. Patients should be advised to report immediately any symptoms of depression and/or suicidal ideation to the prescribing physician. If a patient develops depression, cessation of treatment with Rebif® should be considered.
Hepatic Injury
Severe liver injury, including some cases of hepatic failure requiring liver transplantation, has been reported rarely in patients taking Rebif®. Symptoms of liver dysfunction began from one to six months following the initiation of Rebif®. If jaundice or other symptoms of liver dysfunction appear, treatment with Rebif® should be discontinued immediately due to the potential for rapid progression to liver failure.
Asymptomatic elevation of hepatic transaminases (particularly SGPT) is common with interferon therapy (see ADVERSE REACTIONS). Rebif® should be initiated with caution in patients with active liver disease, alcohol abuse, increased serum SGPT (> 2.5 times ULN), or a history of significant liver disease. Also, the potential risk of Rebif® used in combination with known hepatotoxic products should be considered prior to Rebif® administration, or when adding new agents to the regimen of patients already on Rebif®. Reduction of Rebif® dose should be considered if SGPT rises above 5 times the upper limit of normal. The dose may be gradually re-escalated when enzyme levels have normalized. (See PRECAUTIONS: Laboratory Tests and Drug Interactions; and DOSAGE AND ADMINISTRATION)
Anaphylaxis
Anaphylaxis has been reported as a rare complication of Rebif® use. Other allergic reactions have included skin rash and urticaria, and have ranged from mild to severe without a clear relationship to dose or duration of exposure. Several allergic reactions, some severe, have occurred after prolonged use.
Albumin (Human)
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
PRECAUTIONS
General
Caution should be exercised when administering Rebif® to patients with pre-existing seizure disorders. Seizures have been associated with the use of beta interferons. Leukopenia and new or worsening thyroid abnormalities have developed in some patients treated with Rebif® (see ADVERSE REACTIONS). Regular monitoring for these conditions is recommended (see PRECAUTIONS: Laboratory Tests).
Information for Patients
All patients should be instructed to read the Rebif® Medication Guide supplied to them. Patients should be cautioned not to change the dosage or the schedule of administration without medical consultation.
Patients should be informed of the most common and the most severe adverse reactions associated with the use of Rebif® (see WARNINGS and ADVERSE REACTIONS). Patients should be advised of the symptoms associated with these conditions, and to report them to their physician.
Female patients should be cautioned about the abortifacient potential of Rebif® (see PRECAUTIONS: Pregnancy).
Patients should be instructed in the use of aseptic technique when administering Rebif®. Appropriate instruction for self-injection or injection by another person should be provided to the patient or their caregiver, including careful review of the Rebif® Medication Guide and the Rebif® Rebidose® autoinjector Instructions for Use that accompanies the product. Users should demonstrate competency in all aspects of the injection prior to independent use. If a patient is to self-administer Rebif®, the physical and cognitive ability of that patient to self-administer and properly dispose of pre-filled syringes or the Rebif® Rebidose® autoinjectors should be assessed. Patients with severe neurological deficits should not self administer injections without assistance from a trained caregiver. The initial injection should be performed under the supervision of an appropriately qualified health care provider. Patients can remove the pre-filled syringes or the Rebif® Rebidose® autoinjector from the refrigerator at least 30 minutes prior to use so it can warm to room temperature. Patients should be advised of the importance of rotating sites of injection with each dose, to minimize the likelihood of severe injection site reactions or necrosis and whether or not to pinch the skin prior to injection. A puncture-resistant container for disposal of used needles, pre-filled syringes and Rebif® Rebidose® autoinjectors should be supplied to the patient along with instructions for safe disposal of full containers. Patients should be instructed in the importance of proper disposal of pre-filled syringes and Rebif® Rebidose® autoinjectors and be cautioned against reuse of these items.
Laboratory Tests
In addition to those laboratory tests normally required for monitoring patients with multiple sclerosis, blood cell counts and liver function tests are recommended at regular intervals (1, 3, and 6 months) following introduction of Rebif® therapy and then periodically thereafter in the absence of clinical symptoms. Thyroid function tests are recommended every 6 months in patients with a history of thyroid dysfunction or as clinically indicated. Patients with myelosuppression may require more intensive monitoring of complete blood cell counts, with differential and platelet counts.
Drug Interactions
No formal drug interaction studies have been conducted with Rebif®. Due to its potential to cause neutropenia and lymphopenia, proper monitoring of patients is required if Rebif® is given in combination with myelosuppressive agents.
Also, the potential for hepatic injury should be considered when Rebif® is used in combination with other products associated with hepatic injury, or when new agents are added to the regimen of patients already on Rebif® (see WARNINGS: Hepatic Injury).
Immunization
In a nonrandomized prospective clinical study, 86 multiple sclerosis (MS) patients on Rebif® 44 mcg three times per week for at least 6 months and 77 patients not receiving interferon received influenza vaccination. The proportion of patients achieving a positive antibody response (defined as a titer > 1:40 measured by a hemagglutination inhibition assay) was similar in the two groups (93% and 91%, respectively). The exact relationship of antibody titers to vaccine efficacy was not studied and is not known in patients receiving Rebif®. Therefore, while patients receiving Rebif® may receive concomitant vaccination, the overall effectiveness of such vaccination is unknown.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Rebif® was not mutagenic when tested in the in vitro Ames bacterial test and in an in vitro cytogenetic assay in human lymphocytes in the presence and absence of metabolic activation.
Impairment of Fertility
No studies have been conducted to evaluate the effects of Rebif® on fertility in humans. In studies in normally cycling female cynomolgus monkeys given daily sc injections of Rebif® for six months at doses of up to 9 times the recommended weekly human dose (based on body surface area), no effects were observed on either menstrual cycling or serum estradiol levels. The validity of extrapolating doses used in animal studies to human doses is not established. In male monkeys, the same doses of Rebif® had no demonstrable adverse effects on sperm count, motility, morphology, or function.
Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Rebif® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In a study in pregnant cynomolgus monkeys, Rebif® was administered daily (intramuscular doses approximately 1, 2, and 7 times the maximum recommended cumulative weekly human dose, based on body surface area) either during the period of organogenesis (gestation day 21-89) or later in pregnancy (gestation day 90 to term). In treated dams, non-dose-related increases in spontaneous abortions and fetal and neonatal deaths were observed.
Nursing Mothers
It is not known whether Rebif® is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Rebif® is administered to a nursing woman.
Geriatric Use
Clinical studies of Rebif® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The most frequently reported serious adverse reactions with Rebif® were psychiatric disorders including depression and suicidal ideation or attempt (see WARNINGS). The incidence of depression of any severity in the Rebif®-treated groups and placebo-treated group was approximately 25%. In post-marketing experience, Rebif® administration has been rarely associated with severe liver dysfunction, including hepatic failure requiring liver transplantation (see WARNINGS: Hepatic Injury).
The most commonly reported adverse reactions were injection site disorders, influenza-like symptoms (headache, fatigue, fever, rigors, chest pain, back pain, myalgia), abdominal pain, depression, elevation of liver enzymes and hematologic abnormalities. The most frequently reported adverse reactions resulting in clinical intervention (e.g., discontinuation of Rebif®, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were injection site disorders, influenza-like symptoms, depression and elevation of liver enzymes (see WARNINGS).
In Study 1, 6 patients randomized to Rebif® 44 mcg three times per week (3%), and 2 patients who received Rebif® 22 mcg three times per week (1%) developed injection site necrosis during two years of therapy. Rebif® was continued in 7 patients and interrupted briefly in one patient. There was one report of injection site necrosis in Study 2 during 48 weeks of Rebif® treatment. All events resolved with conservative management; none required skin debridement or grafting.
The rates of adverse reactions and association with Rebif® in patients with relapsing-remitting multiple sclerosis are drawn from the placebo-controlled study (n = 560) and the active comparator-controlled study (n = 339).
The population encompassed an age range from 18 to 55 years. Nearly three-fourths of the patients were female, and more than 90% were Caucasian, largely reflecting the general demographics of the population of patients with multiple sclerosis.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of Rebif® cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
Table 3 enumerates adverse events and laboratory abnormalities that occurred at an incidence that was at least 2% more in either Rebif®-treated group than was observed in the placebo group.
| Body System | Placebo tiw | Rebif® 22 mcg tiw | Rebif® 44 mcg tiw |
|---|---|---|---|
| Preferred Term | (n=187) | (n=189) | (n=184) |
| BODY AS A WHOLE | |||
| Influenza-like symptoms | 51% | 56% | 59% |
| Headache | 63% | 65% | 70% |
| Fatigue | 36% | 33% | 41% |
| Fever | 16% | 25% | 28% |
| Rigors | 5% | 6% | 13% |
| Chest Pain | 5% | 6% | 8% |
| Malaise | 1% | 4% | 5% |
| INJECTION SITE DISORDERS | |||
| Injection Site Reaction | 39% | 89% | 92% |
| Injection Site Necrosis | 0% | 1% | 3% |
| CENTRAL AND PERIPHERAL NERVOUS SYSTEM DISORDERS | |||
| Hypertonia | 5% | 7% | 6% |
| Coordination Abnormal | 2% | 5% | 4% |
| Convulsions | 2% | 5% | 4% |
| ENDOCRINE DISORDERS | |||
| Thyroid Disorder | 3% | 4% | 6% |
| GASTROINTESTINAL SYSTEM DISORDERS | |||
| Abdominal Pain | 17% | 22% | 20% |
| Dry Mouth | 1% | 1% | 5% |
| LIVER AND BILIARY SYSTEM DISORDERS | |||
| SGPT Increased | 4% | 20% | 27% |
| SGOT Increased | 4% | 10% | 17% |
| Hepatic Function Abnormal | 2% | 4% | 9% |
| Bilirubinaemia | 1% | 3% | 2% |
| MUSCULO-SKELETAL SYSTEM DISORDERS | |||
| Myalgia | 20% | 25% | 25% |
| Back Pain | 20% | 23% | 25% |
| Skeletal Pain | 10% | 15% | 10% |
| HEMATOLOGIC DISORDERS | |||
| Leukopenia | 14% | 28% | 36% |
| Lymphadenopathy | 8% | 11% | 12% |
| Thrombocytopenia | 2% | 2% | 8% |
| Anemia | 3% | 3% | 5% |
| PSYCHIATRIC DISORDERS | |||
| Somnolence | 1% | 4% | 5% |
| SKIN DISORDERS | |||
| Rash Erythematous | 3% | 7% | 5% |
| Rash Maculo-Papular | 2% | 5% | 4% |
| URINARY SYSTEM DISORDERS | |||
| Micturition Frequency | 4% | 2% | 7% |
| Urinary Incontinence | 2% | 4% | 2% |
| VISION DISORDERS | |||
| Vision Abnormal | 7% | 7% | 13% |
| Xerophthalmia | 0% | 3% | 1% |
The adverse reactions were generally similar in Studies 1 and 2, taking into account the disparity in study durations.
Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been reported during postmarketing use of Rebif®. Because these reactions were reported voluntarily from a population of uncertain size, the frequency or a causal relationship to Rebif® cannot be reliably determined.
Autoimmune Disorders: Drug-induced lupus erythematosus, autoimmune hepatitis.
Blood and Lymphatic System Disorders: Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), pancytopenia.
Eye Disorders: Retinal vascular disorders (i.e. retinopathy, cotton wool spots or obstruction of retinal artery or vein).
General Disorders and Administration Site Conditions: Increased sweating.
Hepatobiliary Disorders: Rare cases of severe liver dysfunction, including hepatic failure requiring liver transplantation (see WARNINGS: Hepatic Injury).
Nervous System: Seizures (see PRECAUTIONS: General). Transient neurological symptoms (i.e., hypoesthesia, muscle spasm, paresthesia, difficulty walking, musculoskeletal stiffness) that mimic MS exacerbations of limited duration, temporally related to the injections and most prominent at the initiation of therapy. In some cases, these symptoms were associated with flu-like syndrome.
Psychiatric Disorders: Suicide (see WARNINGS: Depression and Suicide).
Skin and Subcutaneous Tissue Disorders: Injection site abscesses, injection site infections, including cellulitis and necrosis requiring debridement, systemic antibiotic treatment and/or grafting; erythema multiforme, and Stevens-Johnson syndrome.
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. In Study 1, the presence of neutralizing antibodies (NAb) to Rebif® was determined by collecting and analyzing serum pre-study and at 6 month time intervals during the 2 years of the clinical trial. Serum NAb were detected in 59/189 (31%) and 45/184 (24%) of Rebif®-treated patients at the 22 mcg and 44 mcg three times per week doses, respectively, at one or more times during the study. The clinical significance of the presence of NAb to Rebif® is unknown.
The data reflect the percentage of patients whose test results were considered positive for antibodies to Rebif® using an antiviral cytopathic effect assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of NAb positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to Rebif® with the incidence of antibodies to other products may be misleading.
Anaphylaxis and other allergic reactions have been observed with the use of Rebif® (see WARNINGS: Anaphylaxis).
DRUG ABUSE AND DEPENDENCE
There is no evidence that abuse or dependence occurs with Rebif® therapy. However, the risk of dependence has not been systematically evaluated.
OVERDOSAGE
Safety of doses higher than 44 mcg sc three times per week has not been adequately evaluated. The maximum amount of Rebif® that can be safely administered has not been determined.
DOSAGE AND ADMINISTRATION
Dosages of Rebif® shown to be safe and effective are 22 mcg and 44 mcg injected subcutaneously three times per week. Rebif® should be administered, if possible, at the same time (preferably in the late afternoon or evening) on the same three days (e.g., Monday, Wednesday, and Friday) at least 48 hours apart each week (see CLINICAL STUDIES). Generally, patients should be started at 20% of the prescribed dose three times per week and increased over a 4-week period to the targeted dose, either 22 mcg three times per week (see Table 4) or 44 mcg three times per week (see Table 5). Patients prescribed a targeted dose of 22 mcg three times per week should use the pre-filled syringes for titration. Following the administration of each dose, any residual product remaining in the syringe should be discarded in a safe and proper manner.
A Titration Pack containing 6 doses of 8.8 mcg (0.2 mL) and 6 doses of 22 mcg (0.5 mL) is available for use during the titration period in both Rebif® pre-filled syringes and Rebif® Rebidose® autoinjectors.
| Week of Use | Dose | Syringe to Use | Amount of syringe |
|---|---|---|---|
|
|||
| Week 1 Titration | 4.4 mcg | 8.8 mcg syringe | Use half of syringe |
| Week 2 Titration | 4.4 mcg | 8.8 mcg syringe | Use half of syringe |
| Week 3 Titration | 11 mcg | 22 mcg syringe | Use half of syringe |
| Week 4 Titration | 11.mcg | 22 mcg syringe | Use half of syringe |
| Week 5 and on | 22 mcg | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week of Use | Dose | Syringe or Autoinjector to Use | Amount of syringe or autoinjector |
|---|---|---|---|
|
|||
| Week 1 Titration | 8.8 mcg | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 2 Titration | 8.8 mcg | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 3 Titration | 22 mcg | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 4 Titration | 22 mcg | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 5 and on | 44 mcg | 44 mcg syringe or autoinjector | Use full syringe or autoinjector |
Leukopenia or elevated liver function tests may necessitate dose reduction or discontinuation of Rebif® administration until toxicity is resolved (see WARNINGS: Hepatic Injury, PRECAUTIONS: General and ADVERSE REACTIONS).
Rebif® is intended for use under the guidance and supervision of a physician. It is recommended that physicians or qualified medical personnel train patients in the proper technique for self-administering subcutaneous (sc) injections using the pre-filled syringe or injection device approved for use with Rebif®. Injection depth of the Rebif® Rebidose® autoinjector is fixed at 8 mm; the health care provider should determine the injection technique. Patients should be advised to rotate sites for sc injections (see PRECAUTIONS: Information for Patients). Concurrent use of analgesics and/or antipyretics may help ameliorate flu-like symptoms on treatment days. Rebif® should be inspected visually for particulate matter and discoloration prior to administration.
Stability and Storage
Rebif® should be stored refrigerated between 36°F to 46°F (2°C to 8°C ). DO NOT FREEZE. If a refrigerator is not available, Rebif® may be stored between 36°F to 77°F (2°C to 25°C) for up to 30 days and away from heat and light.
Do not use beyond the expiration date printed on packages. Rebif® contains no preservatives. Each pre-filled syringe and Rebif® Rebidose® autoinjector is intended for single use. Unused portions should be discarded.
HOW SUPPLIED
Rebif® is supplied as a sterile, preservative-free solution packaged in two different delivery options:
- -
- Pre-filled Syringes: graduated, ready to use in 0.2 mL or 0.5 mL with 29-gauge 0.5 inch needle for subcutaneous injections.
- -
- Rebif® Rebidose® Autoinjectors: pre-assembled, ready to use in 0.2 mL or 0.5 mL with 29-gauge, 0.5 inch needle for subcutaneous injections.
The following package presentations are available:
Pre-Filled Syringes
Rebif® (interferon beta -1a) Titration Pack, NDC 44087-8822-1
- -
- Six Rebif® 8.8 mcg pre-filled syringes and Six Rebif® 22 mcg pre-filled syringes
Rebif® (interferon beta -1a) 22 mcg Pre-filled syringe
- -
- Twelve Rebif® 22 mcg pre-filled syringes, NDC 44087-0022-3
Rebif® (interferon beta -1a) 44 mcg Pre-filled syringe
- -
- Twelve Rebif® 44 mcg pre-filled syringes, NDC 44087-0044-3
Rebif® Rebidose® Autoinjectors
Rebif® (interferon beta-1a) Titration Pack, NDC 44087-0188-1
- -
- Six Rebif® 8.8 mcg autoinjectors with lime-green injector buttons and Six Rebif® 22 mcg with yellow injector buttons.
Rebif® (interferon beta-1a) 22 mcg Rebif® Rebidose® Autoinjector
- -
- Twelve Rebif® 22 mcg autoinjectors with yellow injector buttons, NDC 44087-3322-1
Rebif® (interferon beta-1a) 44 mcg Rebif® Rebidose® Autoinjector
- -
- Twelve Rebif® 44 mcg autoinjectors with teal-green injector buttons, NDC 44087-3344-1
Rx only
References
- PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498-1504.
- Panitch H. Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS. The EVIDENCE Trial. Neurology 2002 59:1496-1506.
Manufactured by EMD Serono, Inc. Rockland, MA 02370 U.S. License # 1773
Marketed by:
EMD Serono, Inc. Pfizer Inc.
Rockland, MA 02370 New York, NY 10017
Revised: February 2013
*Avonex® is a registered trademark of Biogen Idec, Inc.
N67Z0104B
Medication Guide
Rebif® (Re-bif)
interferon beta-1a
(in-ter-feer-on beta-one-â)
Please read this leaflet carefully before you start to use Rebif® and each time your prescription is refilled since there may be new information. The information in this medication guide does not take the place of regularly talking with your doctor or health care provider.
What is the most important information I should know about Rebif®?
Rebif® will not cure multiple sclerosis (MS) but it has been shown to decrease the number of flare-ups and slow the occurrence of some of the physical disability that is common in people with MS. Rebif® can cause serious side effects, so before you start taking Rebif®, you should talk with your doctor about the possible benefits of Rebif® and its possible side effects to decide if Rebif® is right for you. Potential serious side effects include:
- Depression. Some patients treated with interferons, including Rebif®, have become seriously depressed (feeling sad). Some patients have thought about killing themselves and a few have committed suicide. Depression (a sinking of spirits or sadness) is not uncommon in people with multiple sclerosis. However, if you are feeling noticeably sadder or helpless, or feel like hurting yourself or others, you should tell a family member or friend right away and call your doctor as soon as possible. Your doctor may ask that you stop using Rebif®. You should also tell your doctor if you have ever had any mental illness, including depression, and if you take any medications for depression.
- Liver problems. Your liver may be affected by taking Rebif® and a few patients have developed severe liver injury. Your health care provider may ask you to have regular blood tests to make sure that your liver is working properly. If your skin or the whites of your eyes become yellow or if you are bruising easily you should call your doctor right away.
- Risk to pregnancy. If you become pregnant while taking Rebif® you should call your doctor right away. Rebif® may cause you to lose your baby (miscarry) or may cause harm to your unborn child. You and your doctor will need to decide whether the potential benefit of taking Rebif® is greater than the risks are to your unborn child.
- Allergic reactions. Some patients taking Rebif® have had severe allergic reactions leading to difficulty breathing, and loss of consciousness. Allergic reactions can happen after your first dose or may not happen until after you have taken Rebif® many times. Less severe allergic reactions such as itching, flushing or skin bumps can also happen at any time. If you think you are having an allergic reaction, stop using Rebif® immediately and call your doctor.
- Injection site problems. Rebif® may cause redness, pain or swelling at the place where an injection was given. Some patients have developed skin infections or areas of severe skin damage (necrosis) requiring treatment by a doctor. If one of your injection sites becomes swollen and painful or the area looks infected and it doesn't heal within a few days, you should call your doctor.
What is Rebif®?
Rebif® is a type of protein called beta interferon that occurs naturally in the body. It is used to treat relapsing forms of multiple sclerosis. It will not cure your MS but may decrease the number of flare-ups of the disease and slow the occurrence of some of the physical disability that is common in people with MS. MS is a life-long disease that affects your nervous system by destroying the protective covering (myelin) that surrounds your nerve fibers. The way Rebif® works in MS is not known.
Who should not take Rebif®?
Do not take Rebif® if you:
- have had an allergic reaction such as difficulty breathing, flushing or hives to another interferon beta or to human albumin.
If you have any of the following conditions or serious medical problems, you should tell your doctor before taking Rebif®:
- Depression (a sinking feeling or sadness), anxiety (feeling uneasy or fearful for no reason), or trouble sleeping
- Liver diseases
- Problems with your thyroid gland
- Blood problems such as bleeding or bruising easily and anemia (low red blood cells) or low white blood cells
- Epilepsy
- Are planning to become pregnant
Tell your doctor about all medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Rebif® and other medicines may affect each other causing serious side effects. Talk to your doctor before you take any new medicines.
How should I take Rebif®?
Rebif® is given by injection under the skin (subcutaneous injection) on the same three days a week (for example, Monday, Wednesday and Friday). Your injections should be at least 48 hours apart so it is best to take them the same time each day. Your doctor will tell you what dose of Rebif® to use, and may change the dose based on how your body responds. You should not change the dose without talking with your doctor.
If you miss a dose, you should take your next dose as soon as you remember or are able to take it, then skip the following day. Do not take Rebif® on two consecutive days. You should return to your regular schedule the following week. If you accidentally take more than your prescribed dose, or take it on two consecutive days, call your doctor right away.
You should always follow your doctor's instructions and advice about how to take this medication. If your doctor feels that you, or a family member or friend may give you the injections then you and/or the other person should be trained by your doctor or health care provider in how to give an injection. Do not try to give yourself (or have another person give you) injections at home until you (or both of you) understand and are comfortable with how to prepare your dose and give the injections.
Always use a new, unopened, pre-filled syringe of Rebif® or Rebidose® autoinjector for each injection. Do not reuse pre-filled syringes or Rebidose® autoinjectors.
It is important that you change your injection site each time Rebif® is injected. This will lessen the chance of your having a serious skin reaction at the spot where you inject Rebif®. You should always avoid injecting Rebif® into an area of skin that is sore, reddened, infected or otherwise damaged.
At the end of this leaflet, there are detailed instructions on how to prepare and give an injection of Rebif® using a pre-filled syringe. For the Rebidose® autoinjector, refer to the Instructions for Use that comes with the Rebidose® autoinjector. You should become familiar with all instructions and follow your doctor's orders before injecting Rebif®.
What should I avoid while taking Rebif®?
- Pregnancy. You should avoid becoming pregnant while taking Rebif® until you have talked with your doctor. Rebif® can cause you to lose your baby (miscarry).
- Breast feeding. You should talk to your doctor if you are breast feeding an infant. It is not known if the interferon in Rebif® can be passed to an infant in mother's milk, and it is not known whether the drug could harm the infant if it is passed to an infant.
- Rebif® and other medicines may affect each other causing serious side effects. Talk to your doctor before you take any new medicines.
What are the possible side effects of Rebif®?
- Flu-like symptoms. Most patients have flu-like symptoms (fever, chills, sweating, muscle aches and tiredness). For many patients, these symptoms will lessen or go away over time. You should talk to your doctor about whether you should take an over the counter medication for pain or fever reduction before or after taking your dose of Rebif®.
- Skin reactions. Soreness, redness, pain, bruising or swelling may occur at the place of injection. See "What is the most important information I should know about Rebif®?"
- Depression and anxiety. Some patients taking interferons have become very depressed and or anxious. There have been patients taking interferons who have had thoughts about killing themselves. If you feel sad or hopeless you should tell a friend or family member right away and call your doctor immediately. See "What is the most important information I should know about Rebif®?"
- Liver problems. Your liver function may be affected. If you develop symptoms of changes in your liver, including yellowing of the skin and whites of the eyes and easy bruising, call your doctor immediately. See "What is the most important information I should know about Rebif®?"
- Blood problems. You may have a drop in the levels of infection-fighting blood cells, red blood cells or cells that help to form blood clots. If the drop in levels are severe, they can lessen your ability to fight infections, make you feel tired or sluggish or cause you to bruise or bleed easily.
- Thyroid problems: Your thyroid function may change. Symptoms of changes in the function of your thyroid include feeling cold or hot all the time, change in your weight (gain or loss) without a change in your diet or amount of exercise you are getting.
- Allergic reactions: Some patients have had hives, rash, skin bumps or itching while they were taking Rebif®. Other patients have had more serious allergic reactions such as difficulty breathing, or feeling light-headed. You should tell your doctor if you think you are having an allergic reaction. See "What is the most important information I should know about Rebif®?"
Whether you experience any of these side effects or not, you and your doctor should periodically talk about your general health. Your doctor may want to monitor you more closely and ask you to have blood tests done more frequently.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
Storage Conditions
Rebif® is packaged in pre-filled syringes with needles already attached to the syringe and in pre-assembled, single-use autoinjectors, called Rebidose®, with needles already attached within the autoinjector.
Rebif® should be stored refrigerated between 36°F to 46°F (2°C to 8°C). Do Not Freeze. If a refrigerator is not available, Rebif® may be stored between 36°F to 77° °F (2°C to 25°C) for up to 30 days and away from heat and light.
General Information about Prescription Medicines
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. This medication has been prescribed for your particular medical condition. Do not use it for another condition or give this drug to anyone else. If you have any questions you should speak with your doctor or health care provider. You may also ask your doctor or pharmacist for a copy of the information provided to them with the product.
Keep this and all drugs out of the reach of children.
Instructions for Preparing and Giving Yourself an Injection of Rebif® using a Rebidose® autoinjector
Refer to the Instructions for Use that comes with the Rebidose® autoinjector.
Instructions for Preparing and Giving Yourself an Injection of Rebif® using a pre-filled syringe
Before you begin, gather all of the supplies listed below:
- Rebif® pre-filled syringe or Rebidose® autoinjector
- Alcohol swabs (wipes) or cotton balls and rubbing alcohol
- Small adhesive bandage strip (if desired)
- Puncture resistant safety container for disposal of used syringes
- Antibacterial soap
- An over-the-counter pain or fever reducing medication, if your doctor has recommended that you take this prior to, at the same time, or after you give yourself Rebif® to help minimize the fever, chills, sweating and muscle aches (flu-like symptoms) that may occur.
When first starting treatment with Rebif®, your doctor may prescribe either the 22 mcg or 44 mcg dose of Rebif®. You should gradually increase the dose over 4 weeks, starting at 20% of the prescribed dose for the first 2 weeks, half-dose for the second 2 weeks (weeks 3 and 4), and then the full dose prescribed by your doctor.
If your prescribed dose is 22 mcg dose of Rebif®, a Rebif® Titration Pack containing 6 pre-filled syringes with 8.8 mcg and 6 pre-filled syringes with 22 mcg should be prescribed to you for use during the 4 week titration period. Table 1 explains the amount to inject using the Rebif® Titration Pack syringes to gradually increase to 22 mcg.
| Week of Use | Syringe to Use | Amount of syringe |
|---|---|---|
|
||
| Week 1 Titration | 8.8 mcg syringe | Use half of syringe |
| Week 2 Titration | 8.8 mcg syringe | Use half of syringe |
| Week 3 Titration | 22 mcg syringe | Use half of syringe |
| Week 4 Titration | 22 mcg syringe | Use half of syringe |
| Week 5 and on | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
If your prescribed dose is 44 mcg, you may be prescribed either a Rebif® Titration Pack (described above) or Rebif® Rebidose® Titration Pack containing 6 autoinjectors with 8.8 mcg and 6 autoinjectors with 22 mcg for use during the 4 week titration period. Table 2 explains the amount to inject using the Rebif® Titration Pack or Rebif® Rebidose® Titration Pack to gradually increase to 44 mcg.
| Week of Use | Syringe or Autoinjector to Use | Amount of syringe or autoinjector |
|---|---|---|
|
||
| Week 1 Titration | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 2 Titration | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 3 Titration | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 4 Titration | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 5 and on | 44 mcg syringe or autoinjector | Use full syringe or autoinjector |
Preparing for an injection:
- Check the expiration date; do not use if the medication is expired. The expiration date is printed on the syringe, plastic syringe packaging and carton.
- You may wish to remove your medication from the refrigerator at least 30 minutes prior to use so it can warm to room temperature. Do not heat or microwave the medication.
- Be sure that the dose, either, 8.8 mcg, 22 mcg or 44 mcg, described on the carton is the same as the dose prescribed by your doctor.
- Remove the Rebif® syringe or autoinjector from the plastic packaging. Keep the needle capped.
- Examine the contents of the syringe carefully. The liquid should be clear to slightly yellow. Do not use if the liquid is cloudy, discolored or contains particles.
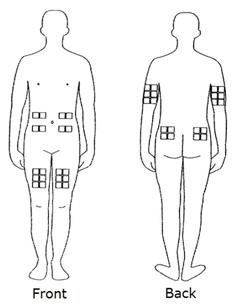
- Choose only one site for injection. The best sites for giving yourself an injection are those areas with a layer of fat between the skin and muscle, like your thigh, the outer surface of your upper arm, your stomach or buttocks. Do not use the area near your waistline or within 2 inches of your navel. If you are very thin, use only the thigh or outer surface of the arm for injection. Use a different site each time you inject (thigh, hip, stomach or upper arm, see Figure below). Do not inject Rebif® into an area of your body where the skin is irritated, reddened, bruised, infected or abnormal in any way.
- Keep a record of the date and location of each injection.
- Wash your hands thoroughly with antibacterial soap before preparing to inject the medication.
- Clean the injection site with an alcohol swab (wipe) or cotton ball with rubbing alcohol using a circular motion. To avoid stinging, you should let your skin dry before you inject Rebif®.
Giving yourself an injection of Rebif® using a pre-filled syringe
- Remove the needle cap from the syringe needle.
- If your doctor has told you to use less than the full 0.5 mL dose, slowly push the plunger in until the amount of medication left in the syringe is the amount your doctor told you to use.
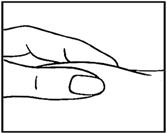
- Use your thumb and forefinger to pinch a pad of skin surrounding the cleaned injection site (see figure below). Hold the syringe like a pencil with your other hand.
- While still pinching the skin, swiftly insert the needle like a dart at about a 90 degree angle (just under the skin) into the pad of tissue as shown.
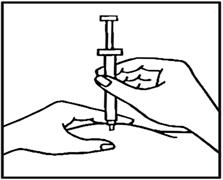
- After the needle is in, remove the hand that you used to pinch your skin and inject the drug using a slow, steady push on the plunger until all the medication is injected and the syringe is empty.
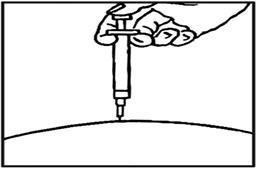
- Withdraw the needle and apply gentle pressure to the injection site with a dry cotton ball or sterile gauze. Applying a cold compress or ice pack to the injection site after injection may help reduce local skin reactions.
- Put a small adhesive bandage strip over the injection site, if desired.
- After 2 hours, check the injection site for redness, swelling, or tenderness. If you have a skin reaction and it doesn't clear up in a few days, contact your doctor or nurse.
Disposing of Needles, Syringes and Rebidose® autoinjectors
There are special state or local laws for properly disposing used needles, syringes and Rebidose® autoinjectors. Your doctor or health care provider will instruct you on the discarding procedure and may provide you with a FDA-cleared disposal container called a Sharps container.
Put your used needles, syringes, or Rebidose® autoinjectors in a Sharps container right away after use. Do not throw away (dispose of) any sharps in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep your disposal container out of the reach of children.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by:
EMD Serono, Inc.
Rockland, MA 02370
U.S. License 1773
Marketed by:
EMD Serono, Inc. Pfizer Inc.
Rockland, MA 02370 New York, NY 10017
December, 2012
N67Z0104B
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 44087-8822-1
Rebif®Titration Pack
(interferon beta-1a)
6 single-use 8.8 mcg/0.2 mL prefilled syringes
6 single-use 22 mcg/0.5 mL prefilled syringes
For subcutaneous injection
Rx only

PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 44087-0188-1
Rebif® Rebidose® Titration Pack
(interferon beta-1a)
Injection
6 single-use 8.8 mcg / 0.2 mL autoinjectors
6 single-use 22 mcg / 0.5 mL autoinjectors
For subcutaneous injection
Rx only
PRINCIPAL DISPLAY PANEL - 12 Single Use Prefilled Syringe Carton
NDC 44087-0022-3
Rebif® 22 mcg/0.5 mL
(interferon beta-1a)
For subcutaneous injection
Rx only

PRINCIPAL DISPLAY PANEL - 12 Single Use Prefilled Syringe Carton
NDC 44087-0044-3
Rebif® 44 mcg/0.5 mL
(interferon beta-1a)
For subcutaneous injection
Rx only

| REBIF
interferon beta-1a kit |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| REBIF REBIDOSE
interferon beta-1a kit |
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
|
||||||||||||||
| REBIF
interferon beta-1a injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| REBIF
interferon beta-1a injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| REBIF REBIDOSE
interferon beta-1a injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| REBIF REBIDOSE
interferon beta-1a injection, solution |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
|||||||||||||
| Labeler - EMD Serono, Inc. (088514898) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Merck Serono. S.A. | 480192579 | MANUFACTURE(44087-8822, 44087-0188, 44087-0022, 44087-0044, 44087-3322, 44087-3344) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Merck Serono, S.p.A | 437803088 | MANUFACTURE(44087-8822, 44087-0188, 44087-0022, 44087-0044, 44087-3322, 44087-3344) | |