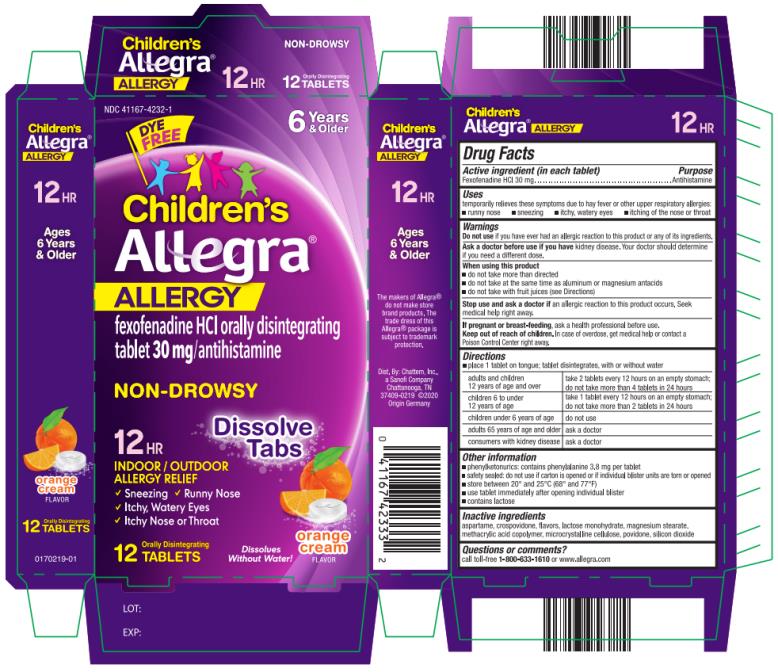
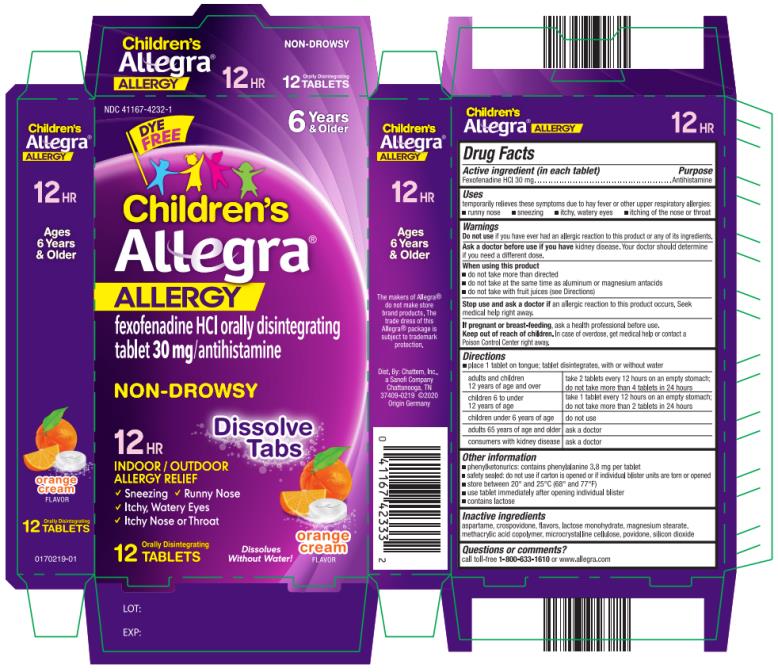
Label: CHILDRENS ALLEGRA ALLERGY- fexofenadine hydrochloride tablet, orally disintegrating
- NDC Code(s): 41167-4232-1, 41167-4232-6
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- do not take more than directed
-
Directions
- place 1 tablet on tongue; tablet disintegrates, with or without water
adults and children 12 years of age and over take 2 tablets every 12 hours on an empty stomach; do not take more than 4 tablets in 24 hours children 6 to under 12 years of age take 1 tablet every 12 hours on an empty stomach; do not take more than 2 tablets in 24 hours children under 6 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLEGRA ALLERGY
fexofenadine hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-4232 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (UNII: 2S7830E561) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor ORANGE (Orange Cream) Imprint Code e;311;AV Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-4232-1 2 in 1 CARTON 01/02/2014 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:41167-4232-6 4 in 1 CARTON 01/02/2014 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021909 01/02/2014 Labeler - Chattem, Inc. (003336013)