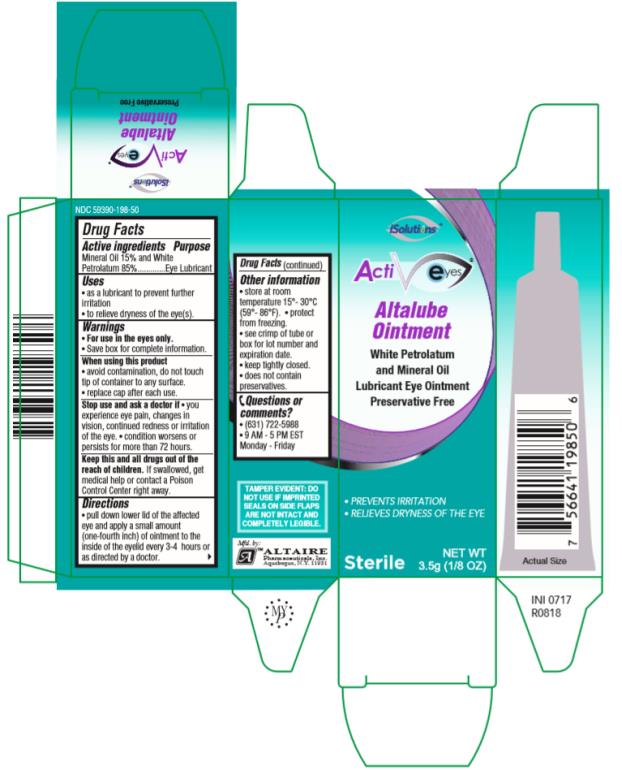
Label: ALTALUBE- mineral oil and petrolatum ointment
- NDC Code(s): 59390-198-50
- Packager: Altaire Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
• Save box for complete information. • For use in the eyes only.
When using this product
• avoid contamination, do not touch tip of container to any surface.
• replace cap after each use.
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALTALUBE
mineral oil and petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59390-198 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 150 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 850 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN ALCOHOLS (UNII: 884C3FA9HE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59390-198-50 1 in 1 CARTON 02/01/2002 1 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/01/2002 Labeler - Altaire Pharmaceuticals Inc. (786790378) Registrant - Altaire Pharmaceuticals, Inc. (786790378) Establishment Name Address ID/FEI Business Operations Altaire Pharmaceuticals, Inc. 786790378 manufacture(59390-198)