Label: PROTOPIC- tacrolimus ointment
-
NDC Code(s):
50222-203-10,
50222-203-30,
50222-203-60,
50222-211-10, view more50222-211-30, 50222-211-60
- Packager: LEO Pharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Rx Only
Prescribing Information
See boxed WARNING concerning long-term safety of topical calcineurin inhibitors
-
DESCRIPTION
Protopic® (tacrolimus) Ointment contains tacrolimus, a macrolide immunosuppressant produced by Streptomyces tsukubaensis. It is for topical dermatologic use only. Chemically, tacrolimus is designated as [3S-[3R*[E(1S*,3S*,4S*)],4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*]]-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10, 12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone,monohydrate. It has the following structural formula:
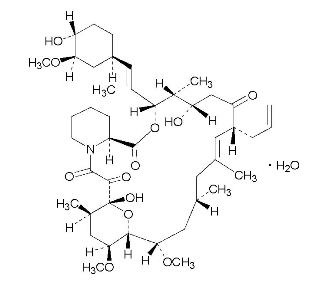
Tacrolimus has an empirical formula of C44H69NO12•H2O and a formula weight of 822.03. Each gram of Protopic® Ointment contains (w/w) either 0.03% or 0.1% of tacrolimus in a base of mineral oil with all-rac-α-tocopherol, paraffin, propylene carbonate, white petrolatum with butylhydroxytoluene, and white wax.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of tacrolimus in atopic dermatitis is not known. While the following have been observed, the clinical significance of these observations in atopic dermatitis is not known. It has been demonstrated that tacrolimus inhibits T-lymphocyte activation by first binding to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is then formed and the phosphatase activity of calcineurin is inhibited. This effect has been shown to prevent the dephosphorylation and translocation of nuclear factor of activated T-cells (NF-AT), a nuclear component thought to initiate gene transcription for the formation of lymphokines (such as interleukin-2, gamma interferon). Tacrolimus also inhibits the transcription for genes which encode IL-3, IL-4, IL-5, GM-CSF, and TNF-α, all of which are involved in the early stages of T-cell activation. Additionally, tacrolimus has been shown to inhibit the release of pre-formed mediators from skin mast cells and basophils, and to down regulate the expression of FcεRI on Langerhans cells.
Pharmacokinetics
Absorption
The pooled results from three pharmacokinetic studies in 88 adult atopic dermatitis patients indicate that tacrolimus is minimally absorbed after the topical application of Protopic® Ointment. Peak tacrolimus blood concentrations ranged from undetectable to 20 ng/mL after single or multiple doses of 0.03% and 0.1% Protopic® Ointment, with 85% (75/88) of the patients having peak blood concentrations less than 2 ng/mL. In general as treatment continued, systemic exposure declined as the skin returned to normal. In clinical studies with periodic blood sampling, a similar distribution of tacrolimus blood levels was also observed in adult patients, with 90% (1253/1391) of patients having a blood concentration less than 2 ng/mL.
The absolute bioavailability of tacrolimus from Protopic® Ointment in atopic dermatitis patients is approximately 0.5%. In adults with an average of 53% BSA treated, exposure (AUC) of tacrolimus from Protopic® Ointment is approximately 30-fold less than that seen with oral immunosuppressive doses in kidney and liver transplant patients.
Mean peak tacrolimus blood concentrations following oral administration (0.3 mg/kg/day) in adult kidney transplant (n=26) and liver transplant (n=17) patients are 24.2±15.8 ng/mL and 68.5±30.0 ng/mL, respectively. The lowest tacrolimus blood level at which systemic effects (e.g., immunosuppression) can be observed is not known.
Systemic levels of tacrolimus have also been measured in pediatric patients (see Special Populations: Pediatrics).
Distribution
The plasma protein binding of tacrolimus is approximately 99% and is independent of concentration over a range of 5-50 ng/mL. Tacrolimus is bound mainly to albumin and alpha-1-acid glycoprotein, and has a high level of association with erythrocytes. The distribution of tacrolimus between whole blood and plasma depends on several factors, such as hematocrit, temperature at the time of plasma separation, drug concentration, and plasma protein concentration. In a US study, the ratio of whole blood concentration to plasma concentration averaged 35 (range 12 to 67).
There was no evidence based on blood concentrations that tacrolimus accumulates systemically upon intermittent topical application for periods of up to 1 year. As with other topical calcineurin inhibitors, it is not known whether tacrolimus is distributed into the lymphatic system.
Metabolism
Tacrolimus is extensively metabolized by the mixed-function oxidase system, primarily the cytochrome P-450 system (CYP3A). A metabolic pathway leading to the formation of 8 possible metabolites has been proposed. Demethylation and hydroxylation were identified as the primary mechanisms of biotransformation in vitro. The major metabolite identified in incubations with human liver microsomes is 13-demethyl tacrolimus. In in vitro studies, a 31-demethyl metabolite has been reported to have the same activity as tacrolimus.
Excretion
The mean clearance following IV administration of tacrolimus is 0.040, 0.083 and 0.053 L/hr/kg in healthy volunteers, adult kidney transplant patients and adult liver transplant patients, respectively. In man, less than 1% of the dose administered is excreted unchanged in urine.
In a mass balance study of IV administered radiolabeled tacrolimus to 6 healthy volunteers, the mean recovery of radiolabel was 77.8 ± 12.7%. Fecal elimination accounted for 92.4 ± 1.0% and the elimination half-life based on radioactivity was 48.1 ± 15.9 hours whereas it was 43.5 ± 11.6 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.029 ± 0.015 L/hr/kg and clearance of tacrolimus was 0.029 ± 0.009 L/hr/kg.
When administered PO, the mean recovery of the radiolabel was 94.9 ± 30.7%. Fecal elimination accounted for 92.6 ± 30.7%, urinary elimination accounted for 2.3 ± 1.1% and the elimination half-life based on radioactivity was 31.9 ± 10.5 hours whereas it was 48.4 ± 12.3 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.226 ± 0.116 L/hr/kg and clearance of tacrolimus 0.172 ± 0.088 L/hr/kg.
Special Populations
Pediatrics
In a pharmacokinetic study of 14 pediatric atopic dermatitis patients, between the ages of 2-5 years, peak blood concentrations of tacrolimus ranged from undetectable to 14.8 ng/mL after single or multiple doses of 0.03% Protopic® Ointment, with 86% (12/14) of patients having peak blood concentrations below 2 ng/mL throughout the study.
The highest peak concentration was observed in one patient with 82% BSA involvement on day 1 following application of 0.03% Protopic® Ointment. The peak concentrations for this subject were 14.8 ng/mL on day 1 and 4.1 ng/mL on day 14. Mean peak tacrolimus blood concentrations following oral administration in pediatric liver transplant patients (n = 9) were 48.4± 27.9 ng/mL.
In a similar pharmacokinetic study with 61 enrolled pediatric patients (ages 6 -12 years) with atopic dermatitis, peak tacrolimus blood concentrations ranged from undetectable to 5.3 ng/mL after single or multiple doses of 0.1% Protopic® Ointment, with 91% (52/57) of evaluable patients having peak blood concentrations below 2 ng/mL throughout the study period. When detected, systemic exposure generally declined as treatment continued.
In clinical studies with periodic blood sampling, a similar distribution of tacrolimus blood levels was also observed, with 98% (509/522) of pediatric patients having a blood concentration below 2 ng/mL.
Renal Insufficiency
The effect of renal insufficiency on the pharmacokinetics of topically administered tacrolimus has not been evaluated. The mean clearance of IV administered tacrolimus in patients with renal dysfunction was similar to that of normal volunteers. On the basis of this information dose-adjustment is not expected to be needed.
Hepatic Insufficiency
The effect of hepatic insufficiency on the pharmacokinetics of topically administered tacrolimus has not been evaluated but dose-adjustment is not expected to be needed.
-
CLINICAL STUDIES
Three randomized, double-blind, vehicle-controlled, multi-center, phase 3 studies were conducted to evaluate Protopic® Ointment for the treatment of patients with moderate to severe atopic dermatitis. One (Pediatric) study included 351 patients 2-15 years of age, and the other two (Adult) studies included a total of 632 patients 15-79 years of age. Fifty-five percent (55%) of the patients were women and 27% were black. At baseline, 58% of the patients had severe disease and the mean body surface area (BSA) affected was 46%. Over 80% of patients had atopic dermatitis affecting the face and/or neck region. In these studies, patients applied either Protopic® Ointment 0.03%, Protopic® Ointment 0.1%, or vehicle ointment twice daily to 10% - 100% of their BSA for up to 12 weeks.
In the pediatric study, a significantly greater (p < 0.001) percentage of patients achieved at least 90% improvement based on the physician’s global evaluation of clinical response (the pre-defined primary efficacy endpoint) in the Protopic® Ointment 0.03% treatment group compared to the vehicle treatment group, but there was insufficient evidence that Protopic® Ointment 0.1% provided more efficacy than Protopic® Ointment 0.03%.
In both adult studies, a significantly greater (p < 0.001) percentage of patients achieved at least 90% improvement based on the physician’s global evaluation of clinical response in the Protopic® Ointment 0.03% and Protopic® Ointment 0.1% treatment groups compared to the vehicle treatment group. There was evidence that Protopic® Ointment 0.1% may provide more efficacy than Protopic® Ointment 0.03%. The difference in efficacy between Protopic® Ointment 0.1% and 0.03% was particularly evident in adult patients with severe disease at baseline, adults with extensive BSA involvement, and black adults. Response rates for each treatment group are shown below by age groups. Because the two adult studies were identically designed, the results from these studies were pooled in this table.
Global Improvement over Baseline at the End-Of-Treatment in Three Phase 3 Studies Physician’s Global Evaluation of Clinical Response
(% Improvement)Pediatric Study (2-15
Years of Age)Adult Studies Vehicle
Ointment
N = 116Protopic® Ointment
0.03%
N = 117Vehicle Ointment
N = 212Protopic® Ointment
0.03%
N = 211Protopic® Ointment
0.1%
N = 209100% 4 (3%) 14 (12%) 2 (1%) 21 (10%) 20 (10%) ≥ 90% 8 (7%) 42 (36%) 14 (7%) 58 (28%) 77 (37%) ≥ 75% 18 (16%) 65 (56%) 30 (14%) 97 (46%) 117 (56%) ≥ 50% 31 (27%) 85 (73%) 42 (20%) 130 (62%) 152 (73%) A statistically significant difference in the percentage of adult patients with ≥ 90% improvement was achieved by week 1 for those treated with Protopic® Ointment 0.1%, and by week 3 for those treated with Protopic® Ointment 0.03%. A statistically significant difference in the percentage of pediatric patients with ≥ 90% improvement was achieved by week 2 for those treated with Protopic® Ointment 0.03%.
In adult patients who had achieved ≥ 90% improvement at the end of treatment, 35% of those treated with Protopic® Ointment 0.03% and 41% of those treated with Protopic® Ointment 0.1%, regressed from this state of improvement at 2 weeks after end-of-treatment. In pediatric patients who had achieved ≥ 90% improvement, 54% of those treated with Protopic® Ointment 0.03% regressed from this state of improvement at 2 weeks after end-of-treatment. Because patients were not followed for longer than 2 weeks after end-of-treatment, it is not known how many additional patients regressed at periods longer than 2 weeks after cessation of therapy.
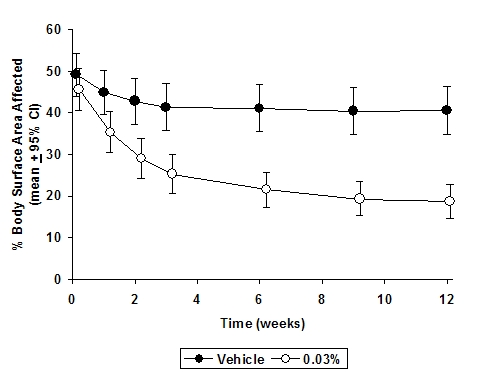
In both Protopic® Ointment treatment groups in adults and in the Protopic® Ointment 0.03% treatment group in pediatric patients, a significantly greater improvement compared to vehicle (p < 0.001) was observed in the secondary efficacy endpoints of percent body surface area involved, patient evaluation of pruritus, erythema, edema, excoriation, oozing, scaling, and lichenification. The following two graphs depict the time course of improvement in the percent body surface area affected in adult and in pediatric patients as a result of treatment.
Figure 1 - Adult Patients Body Surface Area Over Time
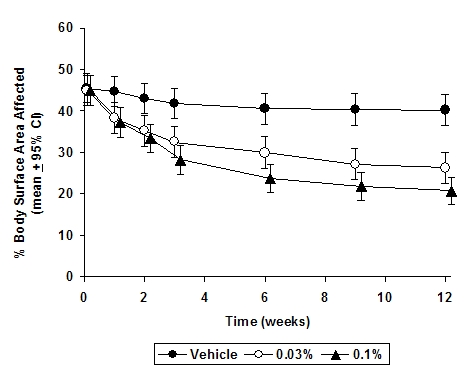
Figure 2 - Pediatric Patients Body Surface Area Over Time
The following two graphs depict the time course of improvement in erythema in adult and in pediatric patients as a result of treatment.
Figure 3 - Adult Patients Mean Erythema Over Time
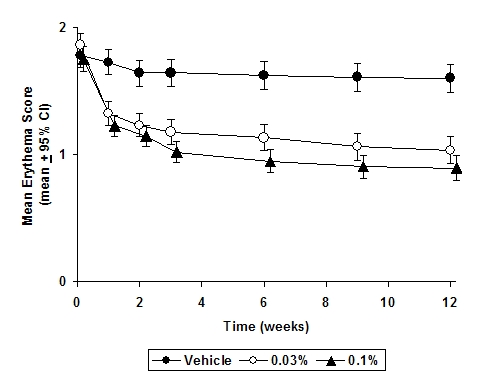
Figure 4 - Pediatric Patients Mean Erythema Over Time
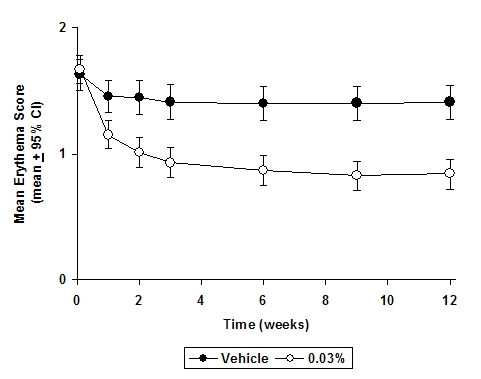
The time course of improvement in the remaining secondary efficacy variables was similar to that of erythema, with improvement in lichenification slightly slower.
-
INDICATIONS AND USAGE
Protopic® Ointment, both 0.03% and 0.1% for adults, and only 0.03% for children aged 2 to 15 years, is indicated as second-line therapy for the short-term and non-continuous chronic treatment of moderate to severe atopic dermatitis in non-immunocompromised adults and children who have failed to respond adequately to other topical prescription treatments for atopic dermatitis, or when those treatments are not advisable.
Protopic® Ointment is not indicated for children younger than 2 years of age (see boxed WARNING, WARNINGS and PRECAUTIONS: Pediatric Use).
- CONTRAINDICATIONS
-
WARNINGS
WARNING
Long-term Safety of Topical Calcineurin Inhibitors Has Not Been Established
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including Protopic® Ointment.
Therefore:
- Continuous long-term use of topical calcineurin inhibitors, including Protopic® Ointment, in any age group should be avoided, and application limited to areas of involvement with atopic dermatitis.
- Protopic® Ointment is not indicated for use in children less than 2 years of age. Only 0.03% Protopic® Ointment is indicated for use in children 2-15 years of age.
Prolonged systemic use of calcineurin inhibitors for sustained immunosuppression in animal studies and transplant patients following systemic administration has been associated with an increased risk of infections, lymphomas, and skin malignancies. These risks are associated with the intensity and duration of immunosuppression.
Based on the information above and the mechanism of action, there is a concern about potential risk with the use of topical calcineurin inhibitors, including Protopic® Ointment. While a causal relationship has not been established, rare cases of skin malignancy and lymphoma have been reported in patients treated with topical calcineurin inhibitors, including Protopic® Ointment. Therefore:
- Protopic® Ointment should not be used in immunocompromised adults and children.
- If signs and symptoms of atopic dermatitis do not improve within 6 weeks, patients should be re-examined by their healthcare provider and their diagnosis be confirmed (see PRECAUTIONS: General).
- The safety of Protopic® Ointment has not been established beyond one year of non-continuous use.
(See CLINICAL PHARMACOLOGY, boxed WARNING, INDICATIONS AND USAGE, and DOSAGE AND ADMINISTRATION).
-
PRECAUTIONS
General
The use of Protopic® Ointment should be avoided on pre-malignant and malignant skin conditions. Some malignant skin conditions, such as cutaneous T-cell lymphoma (CTCL), may mimic atopic dermatitis.
The use of tacrolimus ointment is not recommended in patients having skin conditions with a skin barrier defect where there is the potential for increased systemic absorption of tacrolimus, including but not limited to, Netherton's syndrome, lamellar ichthyosis, generalized erythroderma or cutaneous Graft Versus Host Disease. Oral application is also not recommended. Post-marketing cases of increased tacrolimus blood level have been reported in these conditions.
The use of Protopic® Ointment may cause local symptoms such as skin burning (burning sensation, stinging, soreness) or pruritus. Localized symptoms are most common during the first few days of Protopic® Ointment application and typically improve as the lesions of atopic dermatitis resolve. With Protopic® Ointment 0.1%, 90% of the skin burning events had a duration between 2 minutes and 3 hours (median 15 minutes). 90% of the pruritus events had a duration between 3 minutes and 10 hours (median 20 minutes) (see ADVERSE REACTIONS).
Bacterial and Viral Skin Infections
Before commencing treatment with Protopic® Ointment, cutaneous bacterial or viral infections at treatment sites should be resolved. Studies have not evaluated the safety and efficacy of Protopic® Ointment in the treatment of clinically infected atopic dermatitis.
While patients with atopic dermatitis are predisposed to superficial skin infections including eczema herpeticum (Kaposi’s varicelliform eruption), treatment with Protopic® Ointment may be independently associated with an increased risk of varicella zoster virus infection (chicken pox or shingles), herpes simplex virus infection, or eczema herpeticum.
Patients with Lymphadenopathy
In clinical studies, 112/13494 (0.8%) cases of lymphadenopathy were reported and were usually related to infections (particularly of the skin) and noted to resolve upon appropriate antibiotic therapy. Of these 112 cases, the majority had either a clear etiology or were known to resolve. Transplant patients receiving immunosuppressive regimens (e.g., systemic tacrolimus) are at increased risk for developing lymphoma; therefore, patients who receive Protopic® Ointment and who develop lymphadenopathy should have the etiology of their lymphadenopathy investigated. In the absence of a clear etiology for the lymphadenopathy, or in the presence of acute infectious mononucleosis, Protopic® Ointment should be discontinued. Patients who develop lymphadenopathy should be monitored to ensure that the lymphadenopathy resolves.
Sun Exposure
During the course of treatment, patients should minimize or avoid natural or artificial sunlight exposure, even while Protopic® Ointment is not on the skin. It is not known whether Protopic® Ointment interferes with skin response to ultraviolet damage.
Immunocompromised Patients
The safety and efficacy of Protopic® Ointment in immunocompromised patients have not been studied.
Renal Insufficiency
Rare post-marketing cases of acute renal failure have been reported in patients treated with Protopic® Ointment. Systemic absorption is more likely to occur in patients with epidermal barrier defects especially when Protopic® Ointment is applied to large body surface areas. Caution should also be exercised in patients predisposed to renal impairment.
Information for Patients
(See Medication Guide)
Patients using Protopic® Ointment should receive and understand the information in the Medication Guide. Please refer to the Medication Guide for providing instruction and information to the patient.
What is the most important information patients should know about Protopic® Ointment?
The safety of using Protopic® Ointment for a long period of time is not known. A very small number of people who have used Protopic® Ointment have had cancer (for example, skin or lymphoma). However, a link with Protopic® Ointment has not been shown. Because of this concern, instruct patients:
- Do not use Protopic® Ointment continuously for a long time.
- Use Protopic® Ointment only on areas of skin that have eczema.
- Do not use Protopic® Ointment on a child under 2 years old.
Protopic® Ointment comes in two strengths:
- Only Protopic® Ointment 0.03% is for use on children aged 2 to 15 years.
- Either Protopic® Ointment 0.03% or 0.1% can be used by adults and children 16 years and older.
Advise patients to talk to their prescriber for more information.
How should Protopic® Ointment be used?
Advise patients to:
- Use Protopic® Ointment exactly as prescribed.
- Use Protopic® Ointment only on areas of skin that have eczema.
- Use Protopic® Ointment for short periods, and if needed, treatment may be repeated with breaks in between.
- Stop Protopic® Ointment when the signs and symptoms of eczema, such as itching, rash, and redness go away, or as directed.
- Follow their doctor’s advice if symptoms of eczema return after treatment with Protopic® Ointment.
- Call their doctor if:
- Their symptoms get worse with Protopic® Ointment.
- They get an infection on their skin.
- Their symptoms do not improve after 6 weeks of treatment. Sometimes other skin diseases can look like eczema.
To apply Protopic® Ointment:
Advise patients:
- Wash their hands before applying Protopic® Ointment.
- Apply a thin layer of Protopic® Ointment twice daily to the areas of skin affected by eczema.
- Use the smallest amount of Protopic® Ointment needed to control the signs and symptoms of eczema.
- If they are a caregiver applying Protopic® Ointment to a patient, or if they are a patient who is not treating their hands, wash their hands with soap and water after applying Protopic® Ointment. This should remove any ointment left on the hands.
- Do not bathe, shower, or swim right after applying Protopic® Ointment. This could wash off the ointment.
- Moisturizers can be used with Protopic® Ointment. Make sure they check with their doctor first about the products that are right for them. Because the skin of patients with eczema can be very dry, it is important to keep up good skin care practices. If they use moisturizers, apply them after Protopic® Ointment.
What should patients avoid while using Protopic® Ointment?
Advise patients:
- Do not use ultraviolet light therapy, sun lamps, or tanning beds during treatment with Protopic® Ointment.
- Limit sun exposure during treatment with Protopic® Ointment even when the medicine is not on their skin. If patients need to be outdoors after applying Protopic® Ointment, wear loose fitting clothing that protects the treated area from the sun. Doctors should advise what other types of protection from the sun patients should use.
- Do not cover the skin being treated with bandages, dressings or wraps. Patients can wear normal clothing.
- Avoid getting Protopic® Ointment in the eyes or mouth. Do not swallow Protopic® Ointment. Patients should call their doctor if they swallow Protopic® Ointment.
Drug Interactions
Formal topical drug interaction studies with Protopic®Ointment have not been conducted. Based on its extent of absorption, interactions of Protopic® Ointment with systemically administered drugs are unlikely to occur but cannot be ruled out (see CLINICAL PHARMACOLOGY). The concomitant administration of known CYP3A4 inhibitors in patients with widespread and/or erythrodermic disease should be done with caution. Some examples of such drugs are erythromycin, itraconazole, ketoconazole, fluconazole, calcium channel blockers and cimetidine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of genotoxicity was seen in bacterial (Salmonella and E. coli) or mammalian (Chinese hamster lung-derived cells) in vitro assays of mutagenicity, the in vitro CHO/HGPRT assay of mutagenicity, or in vivo clastogenicity assays performed in mice. Tacrolimus did not cause unscheduled DNA synthesis in rodent hepatocytes.
Oral (feed) carcinogenicity studies have been carried out with systemically administered tacrolimus in male and female rats and mice. In the 80-week mouse study and in the 104-week rat study no relationship of tumor incidence to tacrolimus dosage was found at daily doses up to 3 mg/kg [9X the Maximum Recommended Human Dose (MRHD) based on AUC comparisons] and 5 mg/kg (3X the MRHD based on AUC comparisons), respectively.
A 104-week dermal carcinogenicity study was performed in mice with tacrolimus ointment (0.03% - 3%), equivalent to tacrolimus doses of 1.1-118 mg/kg/day or 3.3-354 mg/m2/day. In the study, the incidence of skin tumors was minimal and the topical application of tacrolimus was not associated with skin tumor formation under ambient room lighting. However, a statistically significant elevation in the incidence of pleomorphic lymphoma in high dose male (25/50) and female animals (27/50) and in the incidence of undifferentiated lymphoma in high dose female animals (13/50) was noted in the mouse dermal carcinogenicity study. Lymphomas were noted in the mouse dermal carcinogenicity study at a daily dose of 3.5 mg/kg (0.1% tacrolimus ointment) (26X MRHD based on AUC comparisons). No drug-related tumors were noted in the mouse dermal carcinogenicity study at a daily dose of 1.1 mg/kg (0.03% tacrolimus ointment) (10X MRHD based on AUC comparisons).
In a 52-week photocarcinogenicity study, the median time to onset of skin tumor formation was decreased in hairless mice following chronic topical dosing with concurrent exposure to UV radiation (40 weeks of treatment followed by 12 weeks of observation) with tacrolimus ointment at ≥0.1% tacrolimus.
Reproductive toxicology studies were not performed with topical tacrolimus. In studies of oral tacrolimus no impairment of fertility was seen in male and female rats. Tacrolimus, given orally at 1.0 mg/kg (0.12X MRHD based on body surface area [BSA]) to male and female rats, prior to and during mating, as well as to dams during gestation and lactation, was associated with embryolethality and with adverse effects on female reproduction. Effects on female reproductive function (parturition) and embryolethal effects were indicated by a higher rate of pre-implantation loss and increased numbers of undelivered and nonviable pups. When given at 3.2 mg/kg (0.43X MRHD based on BSA), tacrolimus was associated with maternal and paternal toxicity as well as reproductive toxicity including marked adverse effects on estrus cycles, parturition, pup viability, and pup malformations.
Pregnancy
Teratogenic Effects:
There are no adequate and well-controlled studies of topically administered tacrolimus in pregnant women. The experience with Protopic® Ointment when used by pregnant women is too limited to permit assessment of the safety of its use during pregnancy.
Reproduction studies were carried out with systemically administered tacrolimus in rats and rabbits. Adverse effects on the fetus were observed mainly at oral dose levels that were toxic to dams. Tacrolimus at oral doses of 0.32 and 1.0 mg/kg (0.04X-0.12X MRHD based on BSA) during organogenesis in rabbits was associated with maternal toxicity as well as an increase in incidence of abortions. At the higher dose only, an increased incidence of malformations and developmental variations was also seen. Tacrolimus, at oral doses of 3.2 mg/kg during organogenesis in rats, was associated with maternal toxicity and caused an increase in late resorptions, decreased numbers of live births, and decreased pup weight and viability. Tacrolimus, given orally at 1.0 and 3.2 mg/kg (0.04X-0.12X MRHD based on BSA) to pregnant rats after organogenesis and during lactation, was associated with reduced pup weights.
No reduction in male or female fertility was evident.
There are no adequate and well-controlled studies of systemically administered tacrolimus in pregnant women. Tacrolimus is transferred across the placenta. The use of systemically administered tacrolimus during pregnancy has been associated with neonatal hyperkalemia and renal dysfunction. Protopic® Ointment should be used during pregnancy only if the potential benefit to the mother justifies a potential risk to the fetus.
Nursing Mothers
Although systemic absorption of tacrolimus following topical applications of Protopic® Ointment is minimal relative to systemic administration, it is known that tacrolimus is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from tacrolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Protopic® Ointment is not indicated for children less than 2 years of age.
Only the lower concentration, 0.03%, of Protopic® Ointment is recommended for use as a second-line therapy for short-term and non-continuous chronic treatment of moderate to severe atopic dermatitis in non-immunocompromised children 2 to 15 years of age who have failed to respond adequately to other topical prescription treatments for atopic dermatitis, or when those treatments are not advisable.
The long-term safety and effects of Protopic® Ointment on the developing immune system are unknown (see boxed WARNING, WARNINGS and INDICATIONS AND USAGE).
Four studies were conducted involving a total of about 4,400 patients 2-15 years of age: one 12-week randomized vehicle-controlled study and three open-label safety studies of one to three years duration. About 2,500 of these patients were 2 to 6 years of age.
The most common adverse events from these studies associated with Protopic® Ointment application in pediatric patients were skin burning and pruritus (see ADVERSE REACTIONS). In addition to skin burning and pruritus, the less common events (<5%) of varicella zoster (mostly chicken pox), and vesiculobullous rash were more frequent in patients treated with Protopic® Ointment 0.03% compared to vehicle. In the open-label safety studies, the incidence of adverse events, including infections, did not increase with increased duration of study drug exposure or amount of ointment used. In about 4,400 pediatric patients treated with Protopic® Ointment, 24 (0.5%) were reported with eczema herpeticum. Since the safety and efficacy of Protopic® Ointment have not been established in pediatric patients below 2 years of age, its use in this age group is not recommended.
In an open-label study, immune response to a 23-valent pneumococcal polysaccharide vaccine was assessed in 23 children 2 to 12 years old with moderate to severe atopic dermatitis treated with Protopic® Ointment 0.03%. Protective antibody titers developed in all patients. Similarly, in a seven-month, double-blind trial, the vaccination response to meningococcal serogroup C was equivalent in children 2 to 11 years old with moderate to severe atopic dermatitis treated with Protopic® Ointment 0.03% (n=121), a hydrocortisone ointment regimen (n=111), or normal children (n=44).
-
ADVERSE REACTIONS
No phototoxicity and no photoallergenicity were detected in clinical studies with 12 and 216 normal volunteers, respectively. One out of 198 normal volunteers showed evidence of sensitization in a contact sensitization study.
In three 12 week randomized vehicle-controlled studies and four safety studies, 655 and 9,163 patients respectively, were treated with Protopic® Ointment. The duration of follow-up for adult and pediatric patients in the safety studies is tabulated below.
Duration of Follow-up in Four Open-label Safety Studies Time on Study Adult Pediatrics Total < 1 year 4682 4481 9163 ≥ 1 year 1185 1349 2534 ≥ 2 years 200 275 475 ≥ 3 years 118 182 300 The following table depicts the adjusted incidence of adverse events pooled across the 3 identically designed 12-week controlled studies for patients in vehicle, Protopic® Ointment 0.03%, and Protopic® Ointment 0.1% treatment groups. The table also depicts the unadjusted incidence of adverse events in four safety studies, regardless of relationship to study drug.
Incidence of Treatment Emergent Adverse Events 12-Week, Randomized, Double-Blind, Phase 3 Studies
12-Week Adjusted Incidence Rate (%)Open-Label Studies
(up to 3 years)
0.1% and 0.03%
Tacrolimus Ointment
Incidence Rate (%)Adult
PediatricAdult Pediatric Total Vehicle
(n=212)
%0.03% Tacrolimus Ointment
(n=210)
%0.1% Tacrolimus Ointment
(n=209)
%Vehicle
(n=116)
%0.03%
Tacrolimus
Ointment
(n=118)
%(n=4682)
%
(n=4481)
%
(n=9163)
%Skin Burning* 26 46 58 29 43 28 20 24 Pruritus* 37 46 46 27 41 25 19 22 Flu-like symptoms* 19 23 31 25 28 22 34 28 Allergic Reaction 8 12 6 8 4 9 13 11 Skin Erythema 20 25 28 13 12 12 7 9 Headache* 11 20 19 8 5 13 9 11 Skin Infection 11 12 5 14 10 9 16 12 Fever 4 4 1 13 21 2 14 8 Infection 1 1 2 9 7 6 10 8 Cough Increased 2 1 1 14 18 3 10 6 Asthma 4 6 4 6 6 4 13 8 Herpes Simplex 4 4 4 2 0 4 3 3 Eczema Herpeticum 0 1 1 0 2 0 0 0 Pharyngitis 3 3 4 11 6 4 12 8 Accidental Injury 4 3 6 3 6 6 8 7 Pustular Rash 2 3 4 3 2 2 7 5 Folliculitis* 1 6 4 0 2 4 2 3 Rhinitis 4 3 2 2 6 2 4 3 Otitis Media 4 0 1 6 12 2 11 6 Sinusitis* 1 4 2 8 3 6 7 6 Diarrhea 3 3 4 2 5 2 4 3 Urticaria 3 3 6 1 1 3 4 4 Lack of Drug Effect 1 1 0 1 1 6 6 6 Bronchitis 0 2 2 3 3 4 4 4 Vomiting 0 1 1 7 6 1 4 3 Maculopapular Rash 2 2 2 3 0 2 1 1 Rash* 1 5 2 4 2 2 3 3 Abdominal Pain 3 1 1 2 3 1 3 2 Fungal Dermatitis 0 2 1 3 0 2 4 3 Gastroenteritis 1 2 2 3 0 2 4 3 Alcohol Intolerance* 0 3 7 0 0 4 0 2 Acne* 2 4 7 1 0 3 2 3 Sunburn 1 2 1 0 0 2 1 1 Skin Disorder 2 2 1 1 4 2 2 2 Conjunctivitis 0 2 2 2 1 3 3 3 Pain 1 2 1 0 1 2 1 2 Vesiculobullous Rash* 3 3 2 0 4 2 1 1 Lymphadenopathy 2 2 1 0 3 1 2 1 Nausea 4 3 2 0 1 2 1 2 Skin Tingling* 2 3 8 1 2 2 1 1 Face Edema 2 2 1 2 1 1 1 1 Dyspepsia* 1 1 4 0 0 2 2 2 Dry Skin 7 3 3 0 1 1 1 1 Hyperesthesia* 1 3 7 0 0 2 0 1 Skin Neoplasm Benign† 1 1 1 0 0 1 2 2 Back Pain* 0 2 2 1 1 3 0 2 Peripheral Edema 2 4 3 0 0 2 0 1 Varicella Zoster/Herpes Zoster*‡ 0 1 0 0 5 1 2 2 Contact Dermatitis 1 3 3 3 4 2 2 2 Asthenia 1 2 3 0 0 1 0 1 Pneumonia 0 1 1 2 0 1 3 2 Eczema 2 2 2 0 0 1 0 1 Insomnia 3 4 3 1 1 2 0 1 Exfoliative Dermatitis 3 3 1 0 0 0 1 0 Dysmenorrhea 2 4 4 0 0 2 1 1 Periodontal Abscess 1 0 1 0 0 1 1 1 Myalgia* 0 3 2 0 0 2 1 1 Cyst* 0 1 3 0 0 1 0 1 Cellulitis 1 1 1 0 0 1 1 1 Exacerbation of Untreated Area 1 0 1 1 0 1 1 1 Procedural Complication 1 0 0 1 0 1 1 1 Hypertension 0 0 1 0 0 2 0 1 Tooth Disorder 0 1 1 1 0 2 1 1 Arthralgia 1 1 3 2 0 2 1 2 Depression 1 2 1 0 0 1 0 1 Paresthesia 1 3 3 0 0 2 1 2 Alopecia 0 1 1 0 0 1 1 1 Urinary Tract Infection
0 0 1 0 0 2 1 2 Ear Pain 1 0 1 0 1 0 1 1 Other adverse events which occurred at an incidence between 0.2% and less than 1% in clinical studies in the above table include: abnormal vision, abscess, anaphylactoid reaction, anemia, anorexia, anxiety, arthritis, arthrosis, bilirubinemia, blepharitis, bone disorder, breast neoplasm benign, bursitis, cataract NOS, chest pain, chills, colitis, conjunctival edema, constipation, cramps, cutaneous moniliasis, cystitis, dehydration, dizziness, dry eyes, dry mouth/nose, dyspnea, ear disorder, ecchymosis, edema, epistaxis, eye pain, furunculosis, gastritis, gastrointestinal disorder, hernia, hypercholesterolemia, hypertonia, hypothyroidism, joint disorder, laryngitis, leukoderma, lung disorder, malaise, migraine, moniliasis, mouth ulceration, nail disorder, neck pain, neoplasm benign, oral moniliasis, otitis externa, photosensitivity reaction, rectal disorder, seborrhea, skin carcinoma, skin discoloration, skin hypertrophy, skin ulcer, stomatitis, tendon disorder, thinking abnormal, tooth caries, sweating, syncope, tachycardia, taste perversion, unintended pregnancy, vaginal moniliasis, vaginitis, valvular heart disease, vasodilatation, and vertigo.
Post-Marketing Events
The following adverse reactions have been identified during postapproval use of Protopic® Ointment. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
CNS
Seizures
Neoplasms
Lymphomas, basal cell carcinoma, squamous cell carcinoma, malignant melanoma
Infections
Bullous impetigo, osteomyelitis, septicemia
Renal
Acute renal failure in patients with or without Netherton’s syndrome, renal impairment
Skin
Rosacea, application site edema
-
DOSAGE AND ADMINISTRATION
ADULT
Protopic® Ointment 0.03% and 0.1%
- Apply a thin layer of Protopic® Ointment to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of atopic dermatitis. Stop using when signs and symptoms of atopic dermatitis resolve.
- If signs and symptoms (e.g. itch, rash, and redness) do not improve within 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis.
- Continuous long-term use of topical calcineurin inhibitors, including Protopic® Ointment should be avoided, and application should be limited to areas of involvement with atopic dermatitis.
The safety of Protopic® Ointment under occlusion, which may promote systemic exposure, has not been evaluated. Protopic® Ointment should not be used with occlusive dressings.
PEDIATRIC – FOR CHILDREN 2-15 YEARS
Protopic® Ointment 0.03%
- Apply a thin layer of Protopic® Ointment, 0.03% to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of atopic dermatitis. Stop using when signs and symptoms of atopic dermatitis resolve.
- If signs and symptoms (e.g. itch, rash, and redness) do not improve within 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis.
- Continuous long-term use of topical calcineurin inhibitors, including Protopic® Ointment should be avoided, and application should be limited to areas of involvement with atopic dermatitis.
The safety of Protopic® Ointment under occlusion, which may promote systemic exposure, has not been evaluated. Protopic® Ointment should not be used with occlusive dressings.
-
HOW SUPPLIED
Protopic® Ointment 0.03%
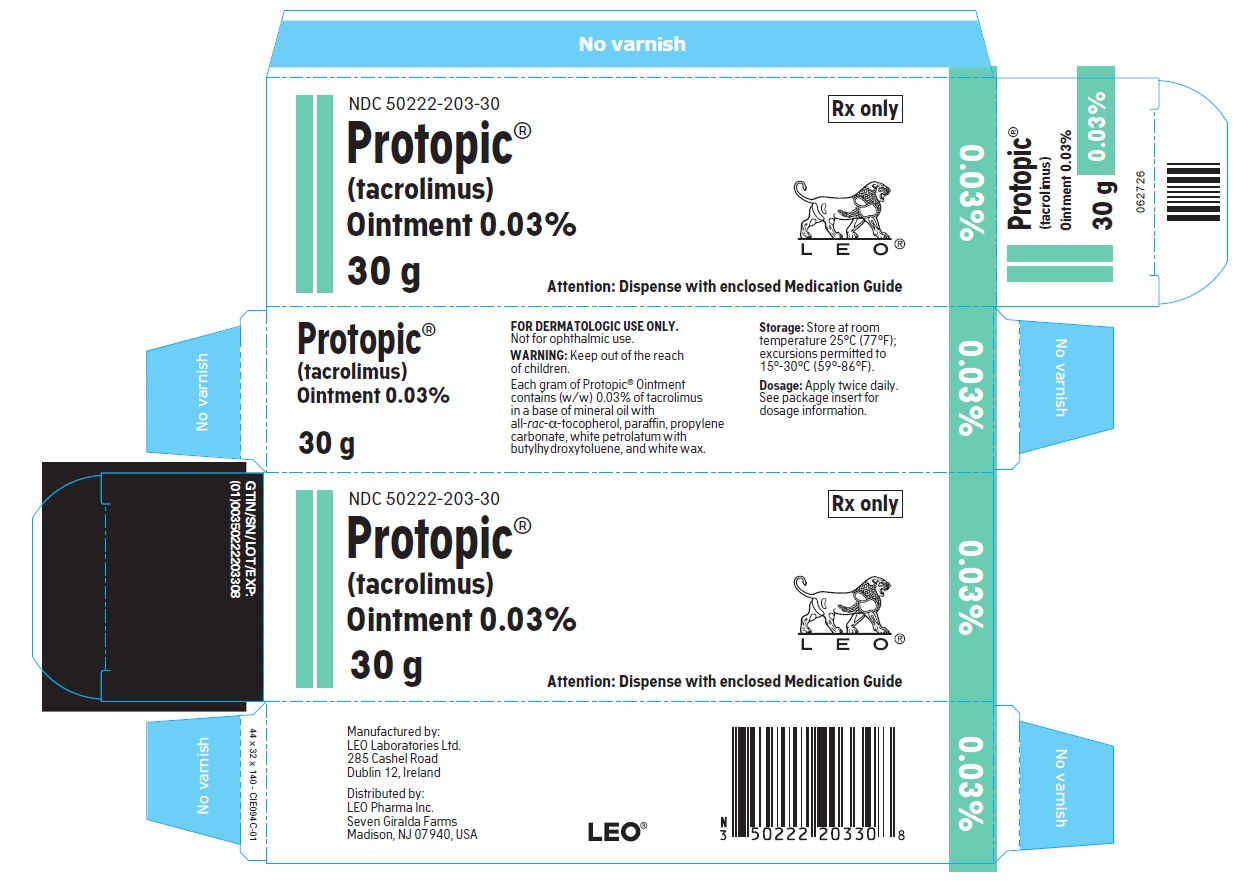
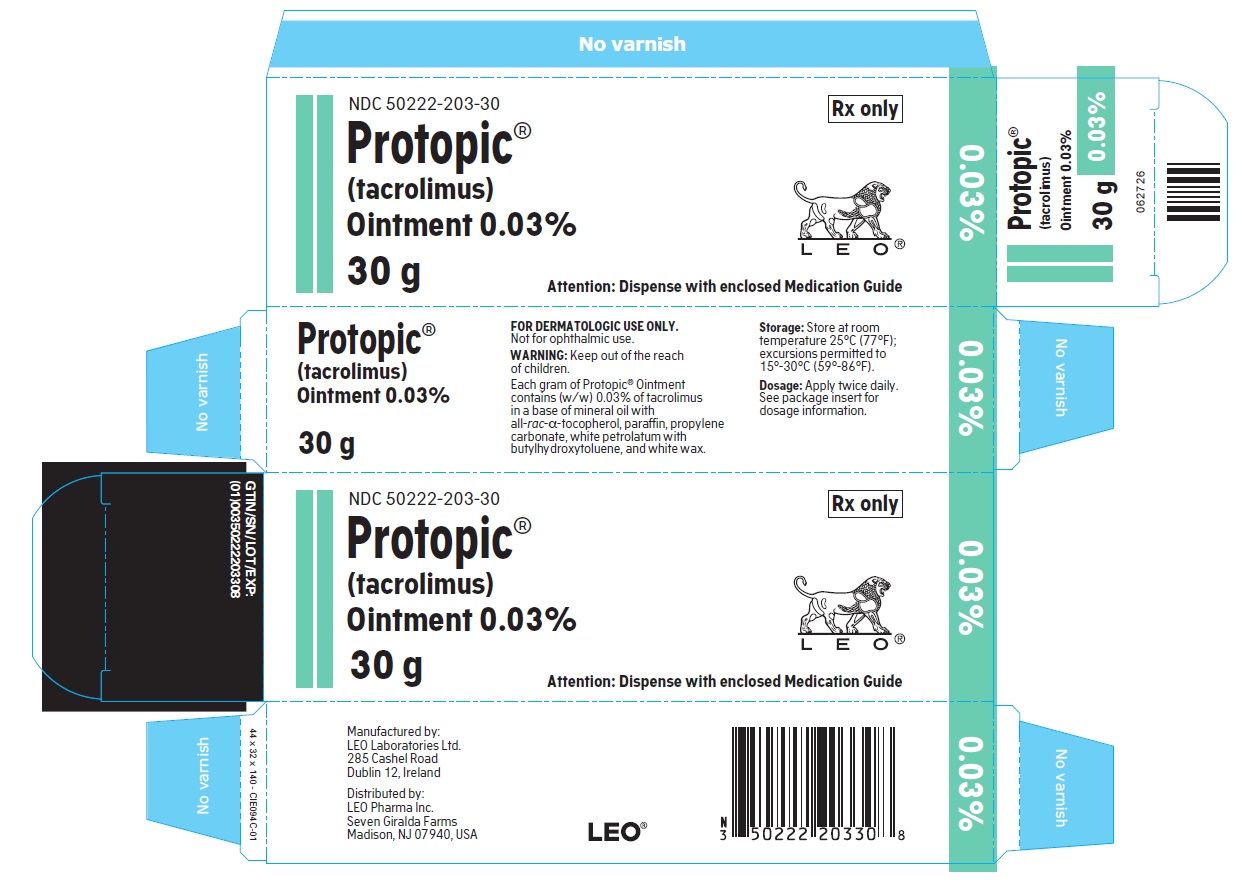
NDC 50222-203-30
30 gram laminate tube
NDC 50222-203-60
60 gram laminate tube
NDC 50222-203-10
100 gram laminate tube
Protopic® Ointment 0.1%
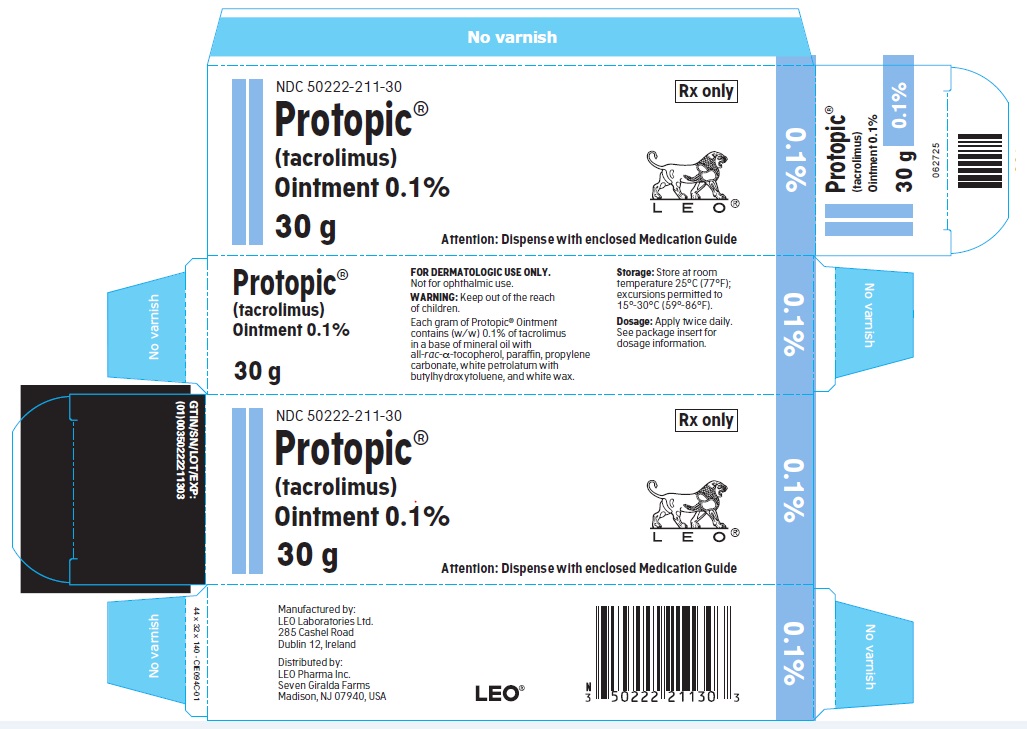
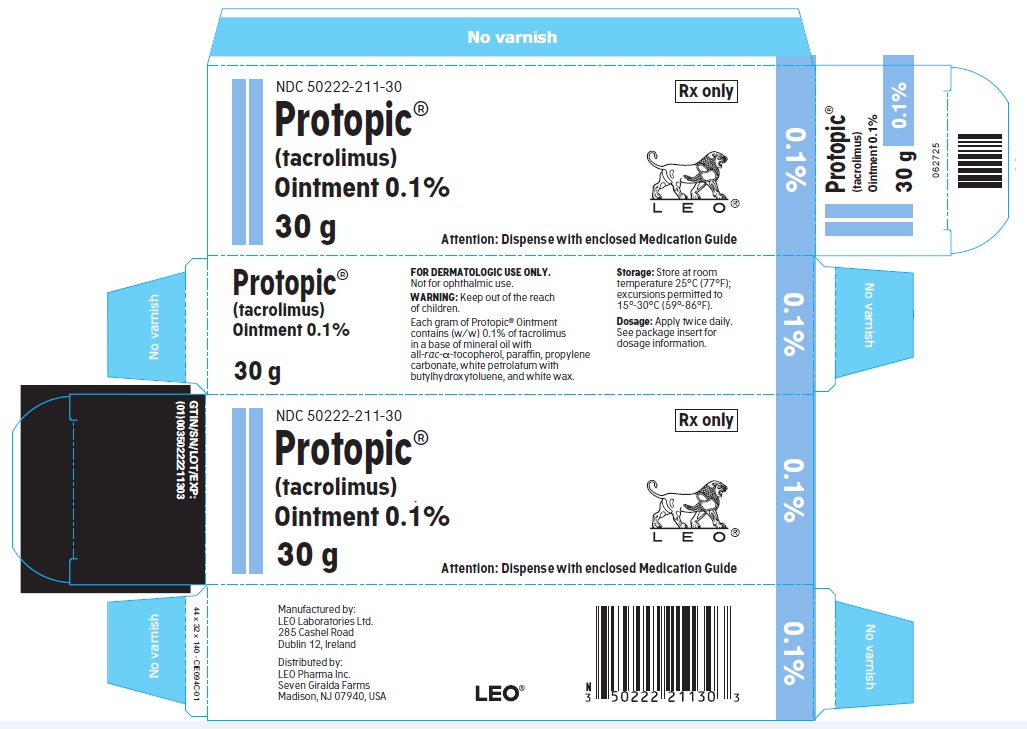
NDC 50222-211-30
30 gram laminate tube
NDC 50222-211-60
60 gram laminate tube
NDC 50222-211-10
100 gram laminate tube
Store at room temperature 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
Manufactured by:
LEO®

LEO Laboratories Ltd.
285 Cashel Road
Dublin 12, Ireland
Distributed by:LEO Pharma Inc.
Seven Giralda Farms
Madison, NJ 07940, USA
Revised: 02/2019
-
MEDICATION GUIDE
Protopic® [pro-TOP-ik]
(tacrolimus)
Ointment 0.03%
Ointment 0.1%
Read the Medication Guide every time you or a family member gets Protopic® (tacrolimus) Ointment. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment. If you have questions about Protopic® Ointment, ask your doctor or pharmacist.
What is the most important information I should know about Protopic® Ointment?
The safety of using Protopic® Ointment for a long period of time is not known. A very small number of people who have used Protopic® Ointment have had cancer (for example, skin or lymphoma). However, a link with Protopic® Ointment has not been shown. Because of this concern:
- Do not use Protopic® Ointment continuously for a long time.
- Use Protopic® Ointment only on areas of your skin that have eczema.
- Do not use Protopic® Ointment on a child under 2 years old.
Protopic® Ointment comes in two strengths:
- Only Protopic® Ointment 0.03% is for use on children aged 2 to 15 years.
- Either Protopic® Ointment 0.03% or 0.1% can be used by adults and children 16 years and older.
Talk to your doctor for more information.
What is Protopic® Ointment?
Protopic® Ointment is a prescription medicine used on the skin (topical) to treat eczema (atopic dermatitis). Protopic® Ointment is in a class of medicines called topical calcineurin inhibitors. It is for adults and children 2 years of age and older who do not have a weakened immune system. Protopic® Ointment is used on the skin for short periods, and if needed, treatment may be repeated with breaks in between.
Protopic® Ointment is for use after other prescription medicines have not worked for you, or if your doctor recommends that other prescription medicines should not be used.
Who should not use Protopic® Ointment?
Protopic® Ointment should not be used:
- on children younger than 2 years of age.
- if you are allergic to Protopic® Ointment or anything in it. See the end of this Medication Guide for a complete list of ingredients.
What should I tell my doctor before starting Protopic® Ointment?
Before you start using Protopic® Ointment, you and your doctor should talk about all of your medical conditions, including if you:
- have a skin disease called Netherton’s syndrome (a rare inherited condition).
- have any infection on your skin including chicken pox or herpes.
- have been told you have a weakened immune system.
- are pregnant, breastfeeding, or planning to become pregnant.
Tell your doctor about all the medicines you take and skin products you use including prescription and nonprescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I use Protopic® Ointment?
- Use Protopic® Ointment exactly as prescribed.
- Use Protopic® Ointment only on areas of your skin that have eczema.
- Use Protopic® Ointment for short periods, and if needed, treatment may be repeated with breaks in between.
- Stop Protopic® Ointment when the signs and symptoms of eczema, such as itching, rash, and redness go away, or as directed by your doctor.
- Follow your doctor’s advice if symptoms of eczema return after treatment with Protopic® Ointment.
- Call your doctor if:
- your symptoms get worse with Protopic® Ointment.
- you get an infection on your skin.
- your symptoms do not improve after 6 weeks of treatment. Sometimes other skin diseases can look like eczema.
To apply Protopic® Ointment:
- Wash your hands before applying Protopic® Ointment.
- Apply a thin layer of Protopic® Ointment twice daily to the areas of skin affected by eczema.
- Use the smallest amount of Protopic® Ointment needed to control the signs and symptoms of eczema.
- If you are a caregiver applying Protopic® Ointment to a patient, or if you are a patient who is not treating your hands, wash your hands with soap and water after applying Protopic® Ointment. This should remove any ointment left on the hands.
- Do not bathe, shower, or swim right after applying Protopic® Ointment. This could wash off the ointment.
- You can use moisturizers with Protopic® Ointment. Make sure you check with your doctor first about the products that are right for you. Because the skin of patients with eczema can be very dry, it is important to keep up good skin care practices. If you use moisturizers, apply them after Protopic® Ointment.
What should I avoid while using Protopic® Ointment?
- Do not use ultraviolet light therapy, sun lamps, or tanning beds during treatment with Protopic® Ointment.
- Limit sun exposure during treatment with Protopic® Ointment even when the medicine is not on your skin. If you need to be outdoors after applying Protopic® Ointment, wear loose fitting clothing that protects the treated area from the sun. Ask your doctor what other types of protection from the sun you should use.
- Do not cover the skin being treated with bandages, dressings or wraps. You can wear normal clothing.
- Avoid getting Protopic® Ointment in the eyes or mouth. Do not swallow Protopic® Ointment. If you do, call your doctor.
What are the possible side effects of Protopic® Ointment?
Please read the first section of this Medication Guide.
The most common side effects of Protopic® Ointment at the skin application site are stinging, burning, or itching of the skin treated with Protopic® Ointment. These side effects are usually mild to moderate, are most common during the first few days of treatment, and usually go away as your skin heals.
Other side effects include acne, swollen or infected hair follicles, headache, increased sensitivity of the skin to hot or cold temperatures, or flu-like symptoms such as the common cold and stuffy nose, skin tingling, upset stomach, muscle pain, swollen glands (enlarged lymph nodes), or skin infections including cold sores, chicken pox or shingles.
Talk to your doctor if you have a skin infection or if side effects (for example, swollen glands) continue or bother you.
While you are using Protopic® Ointment, drinking alcohol may cause the skin or face to become flushed or red and feel hot.
These are not all the side effects with Protopic® Ointment. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at: 1-800-FDA-1088.
How should I store Protopic® Ointment?
- Store Protopic® Ointment at room temperature (59° to 86°F). Do not leave Protopic® Ointment in your car in cold or hot weather. Make sure the cap on the tube is tightly closed.
- Keep Protopic® Ointment and all medicines out of the reach of children.
General advice about Protopic® OintmentMedicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Protopic® Ointment for a condition for which it was not prescribed. Do not give Protopic® Ointment to other people, even if they have the same symptoms you have. It may not be right for them.
This Medication Guide summarizes the most important information about Protopic® Ointment. If you would like more information, talk with your doctor.
Your doctor or pharmacist can give you information about Protopic® Ointment that is written for health care professionals. For more information, you can call: 1-877-494-4536.
What are the ingredients in Protopic® Ointment?
Active Ingredient: tacrolimus, either 0.03% or 0.1%
Inactive Ingredients: mineral oil, paraffin, propylene carbonate, white petrolatum, white wax, all-rac-α-tocopherol and butylhydroxytoluene.
LEO®

Manufactured by:
LEO Laboratories Ltd.
285 Cashel Road
Dublin 12, Ireland
Distributed by:
LEO Pharma Inc.
Seven Giralda Farms
Madison, NJ 07940, USA
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised: 02/2019
- PRINCIPLE DISPLAY PANEL FOR PROTOPIC® (TACROLIMUS) OINTMENT 0.03%
- PRINCIPLE DISPLAY PANEL FOR PROTOPIC® (TACROLIMUS) OINTMENT 0.1%
-
INGREDIENTS AND APPEARANCE
PROTOPIC
tacrolimus ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50222-203 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TACROLIMUS (UNII: WM0HAQ4WNM) (TACROLIMUS ANHYDROUS - UNII:Y5L2157C4J) TACROLIMUS ANHYDROUS 0.3 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PETROLATUM (UNII: 4T6H12BN9U) WHITE WAX (UNII: 7G1J5DA97F) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50222-203-30 1 in 1 CARTON 08/19/2016 08/31/2024 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:50222-203-60 1 in 1 CARTON 08/19/2016 08/31/2024 2 60 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:50222-203-10 1 in 1 CARTON 08/19/2016 05/31/2024 3 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050777 12/08/2000 12/31/2024 PROTOPIC
tacrolimus ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50222-211 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TACROLIMUS (UNII: WM0HAQ4WNM) (TACROLIMUS ANHYDROUS - UNII:Y5L2157C4J) TACROLIMUS ANHYDROUS 1 mg in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50222-211-30 1 in 1 CARTON 08/19/2016 12/31/2024 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:50222-211-60 1 in 1 CARTON 08/19/2016 08/31/2024 2 60 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:50222-211-10 1 in 1 CARTON 08/19/2016 08/31/2024 3 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050777 12/08/2000 12/31/2024 Labeler - LEO Pharma Inc (832692615) Registrant - LEO Pharma A/S (306218108)