Label: RIXUBIS (coagulation factor ix- recombinant kit
-
NDC Code(s):
0944-3025-01,
0944-3026-02,
0944-3027-01,
0944-3028-02, view more0944-3029-01, 0944-3030-02, 0944-3031-01, 0944-3032-02, 0944-3033-01, 0944-3034-02, 64764-515-50
- Packager: Takeda Pharmaceuticals America, Inc.
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RIXUBIS safely and effectively. See full prescribing information for RIXUBIS.
RIXUBIS [Coagulation Factor IX (Recombinant)]
For Intravenous Injection, Lyophilized Powder for Solution
Initial U.S. Approval: 2013INDICATIONS AND USAGE
RIXUBIS (Coagulation Factor IX [Recombinant]) is an antihemophilic factor indicated in adults and children with hemophilia B for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes.
RIXUBIS is not indicated for induction of immune tolerance in patients with Hemophilia B. (1)
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only. (2)
On-demand treatment and control of bleeding episodes and perioperative management of bleeding:
- One international unit of RIXUBIS per kg of body weight increases the circulating activity of factor IX by 0.7 international units/dL for patients <12 years of age and 0.9 international units/dL for patients ≥12 years of age. (2.1)
Initial Dose:
- Required international units = body weight (kg) × desired factor IX increase (% of normal or IU/dL) × reciprocal of observed recovery (IU/kg per IU/dL). (2.1)
- The maintenance dose depends on the type of bleed or surgery, the intensity of the hemostatic challenge, and number of days until adequate wound healing is achieved. (2.2)
Routine prophylaxis:
- Patients <12 years of age: 60 to 80 international units per kg twice weekly.
- Patients ≥12 years of age: 40 to 60 international units per kg twice weekly. (2.1)
DOSAGE FORMS AND STRENGTHS
RIXUBIS is available as a lyophilized powder in single-dose vials containing nominally 250, 500, 1000, 2000 or 3000 international units. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions, including anaphylaxis, may occur. Should symptoms occur, discontinue RIXUBIS and administer appropriate treatment. Patients may also develop hypersensitivity to hamster (CHO) protein, which is present in trace amounts in the product. (5.1)
- Development of neutralizing antibodies (inhibitors) to RIXUBIS may occur. If expected factor IX activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor IX inhibitor concentration. (5.2)
- Nephrotic syndrome has been reported following immune tolerance induction with factor IX products in hemophilia B patients with factor IX inhibitors. (5.3)
- The use of factor IX-containing products has been associated with the development of thromboembolic complications. (5.4)
ADVERSE REACTIONS
Common adverse reactions observed in >1% of subjects in clinical trials were: dysgeusia, pain in extremity, and positive test for furin antibody. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatric Use: A 22% lower recovery has been observed in pediatric patients (<12 years, n=23). Clearance (based on per kg body weight) was 30% (6-12 years) and 59% (<6 years) higher in children than adults. Dose adjustment is needed. (2.1, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
2.2 On-demand Treatment and Control of Bleeding Episodes and Perioperative Management of Bleeding
2.3 Routine Prophylaxis
2.4 Preparation and Reconstitution
2.5 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Inhibitors
5.3 Nephrotic Syndrome
5.4 Thromboembolic Complications
5.5 Monitoring Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
RIXUBIS (Coagulation Factor IX [Recombinant]) is an antihemophilic factor indicated in adults and children with hemophilia B for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes.
RIXUBIS is not indicated for induction of immune tolerance in patients with hemophilia B [see Warnings and Precautions (5.3)].
-
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
- Each vial of RIXUBIS has the recombinant Factor IX (rFIX) potency in international units stated on the vial.
- Initiate treatment under the supervision of a physician experienced in the treatment of hemophilia.
- Dosage and duration of treatment with RIXUBIS depend on the severity of factor IX deficiency, the location and extent of bleeding, the patient's clinical condition, age, and pharmacokinetic parameters of factor IX, such as incremental recovery and half-life.
- Dosing of RIXUBIS may differ from that of plasma-derived factor IX products [see Clinical Pharmacology (12)]. Subjects at the low end of the observed factor IX recovery range may require dose adjustment of RIXUBIS.
- Monitor patients using a factor IX activity assay to ensure that the desired factor IX activity plasma level has been attained. If necessary, adjust the dose and the frequency of repeated infusions as appropriate.
- Evaluate the patient for the development of factor IX inhibitors if the expected factor IX activity plasma levels are not attained or if bleeding is not controlled with an appropriate dose [see Warnings and Precautions (5.2)].
2.1 Dosing Guidelines
Calculating Initial Dose
The initial dose of RIXUBIS is calculated based on the empirical finding that one international unit of RIXUBIS per kg body weight is expected to increase the circulating level of factor IX by 0.7 international units/dL of plasma (0.7% of normal) for patients <12 years of age and by 0.9 international units/dL of plasma (0.9% of normal) in patients ≥12 years of age.
A guide for calculating the initial dose of RIXUBIS for treatment of bleeding episodes is as follows:
Initial Dose = body weight (kg) × desired factor IX increase (% of normal or IU/dL) × reciprocal of observed recovery (IU/kg per IU/dL) Incremental Recovery in Previously Treated Patients (PTPs)
Base the calculation of the dose on the patient's individual incremental recovery using serial factor IX activity assays, due to the wide range of inter-individual differences in incremental recovery. Titrate the dose based on the patient's clinical response and individual pharmacokinetics, in particular incremental recovery and half-life.
Patients <12 Years of Age
On average, a 22% lower recovery has been observed in pediatric patients (<12 years, n=23). For an incremental recovery of 0.7 international units/dL of plasma (0.7% of normal), the dose is calculated as follows:
Dose (international units) = body weight (kg) × desired factor IX increase (% of normal or IU/dL) × 1.4 dL/kg Example (assuming patient's baseline factor IX level is <1% of normal)
- A dose of 1500 international units of RIXUBIS, administered to a 20 kg patient should be expected to result in a peak post-infusion factor IX increase of 1500 international units × {[0.7 IU/dL]/[IU/kg]}/[20 kg] = 53.6 international units/dL (53.6% of normal).
Patients ≥12 Years of Age
For an incremental recovery of 0.9 international units/dL of plasma (0.9% of normal), the dose is calculated as follows:
Dose (international units) = body weight (kg) × desired factor IX increase (% of normal or IU/dL) × 1.1 dL/kg Examples (assuming patient's baseline factor IX level is <1% of normal):
- A dose of 4550 international units of RIXUBIS, administered to a 70 kg patient, should be expected to result in a peak post-infusion factor IX increase of 4550 international units × {[0.9 IU/dL]/[IU/kg]}/[70 kg] = 58.5 international units/dL (58.5 % of normal).
- A peak level of 70% is required in a 60 kg patient. The appropriate dose would be 60 kg × 70 international units/dL/{[0.9 IU/dL]/[IU/kg]} = 4667 international units.
2.2 On-demand Treatment and Control of Bleeding Episodes and Perioperative Management of Bleeding
A guide for dosing RIXUBIS in the on-demand treatment and control of bleeding episodes and perioperative management of bleeding is provided in Table 1 and Table 2, respectively. Ensure the factor IX activity level is achieved and maintained in the corresponding period.
Table 1 Dosing for On-demand Treatment and Control of Bleeding Episodes1 Type of Bleeding Episodes Circulating Factor IX Level Required (% or IU/dL) Dosing Interval (hours) Duration of Therapy (days) Adapted from Roberts and Eberst1 Minor
Uncomplicated hemarthrosis, superficial muscular or soft tissue20-30 12-24 At least 1 day, until healing is achieved Moderate
Intramuscular or soft tissue with dissection, mucous membranes, hematuria25-50 12-24 2-7 days, until bleeding stops, and healing is achieved Major
Pharyngeal, retropharyngeal, retroperitoneal, CNS50-100 12-24 7-10 days, until bleeding stops, and healing is achieved Table 2 Dosing for Perioperative Management of Bleeding Type of Surgery Circulating Factor IX Level Required (% or IU/dL) Dosing Interval (hours) Duration of Therapy (days) Minor
e.g., tooth extraction30-60 24 At least 1 day, until healing is achieved Major
e.g., intracranial, intraabdominal, intrathoracic, joint replacement80-100 8-24 7-10 days, until bleeding stops and healing is achieved 2.3 Routine Prophylaxis
The dose for previously treated patients (PTPs) is 60 to 80 international units per kg twice weekly for patients <12 years of age and is 40 to 60 international units per kg twice weekly for patients ≥12 years of age. Adjust the dose based on the individual patient's age, bleeding pattern, and physical activity.
2.4 Preparation and Reconstitution
The procedures below are provided as general guidelines for the preparation and reconstitution of RIXUBIS. Always work on a clean surface and wash hands before performing the following procedures:
- Use aseptic technique during reconstitution procedure.
- Allow the RIXUBIS vial (dry factor concentrate) and Sterile Water for Injection, USP vial (diluent) to reach room temperature.
- Remove caps from the factor concentrate and diluent vials.
- Cleanse stoppers with germicidal solution and allow the stopper to dry prior to use. Place the vials on a flat surface.
- Open the BAXJECT II device package by peeling away the lid, without touching the inside (Figure A). Do not remove the device from the package. Note that the BAXJECT II device is intended for use with a single vial of RIXUBIS and Sterile Water for Injection, USP only; therefore, reconstituting and withdrawing a second vial into the syringe requires a second BAXJECT II device.
- Turn the package over. Press straight down to fully insert the clear plastic spike through the diluent vial stopper (Figure B).
- Grip the BAXJECT II package at its edge and pull the package off the device (Figure C). Do not remove the blue cap from the BAXJECT II device. Do not touch the exposed white plastic spike.
- Turn the system over so that the diluent vial is on top. Quickly insert the white plastic spike fully into the RIXUBIS vial stopper by pushing straight down (Figure D). The vacuum will draw the diluent into the RIXUBIS vial.
- Swirl gently until the powder is completely dissolved. Do not refrigerate after reconstitution. Use within 3 hours of reconstitution.
2.5 Administration
For intravenous bolus infusion only.
- The safety and efficacy of RIXUBIS administration by continuous infusion has not been established.
- Inspect parenteral drug products for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance. Do not use RIXUBIS if you notice any particulates or turbidity in the solution and notify Takeda Pharmaceuticals.
- Perform product administration and handling of the administration set and needles with caution. Percutaneous puncture with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. Obtain immediate medical attention if injury occurs. Place needles in a sharps container after single-use.
- Administer RIXUBIS at room temperature and within 3 hours of reconstitution. Discard any unused product.
- Use a plastic syringe with this product.
- Remove the blue cap from the BAXJECT II device. Connect the syringe to the BAXJECT II device by screwing it clockwise until the syringe is secured (Figure E). Do not over tighten.
- Do not inject air.
- Turn the system upside down (factor concentrate vial now on top). Draw the factor concentrate into the syringe by pulling the plunger back slowly (Figure F).
- Disconnect the syringe by unscrewing it counterclockwise; attach a suitable needle to the syringe and inject intravenously by bolus infusion. If a patient is to receive more than one vial of RIXUBIS, the contents of multiple vials may be drawn into the same syringe.
- Maximum infusion rate of 10 mL/min.
Figure A 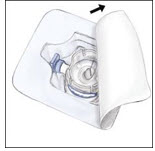
Figure B 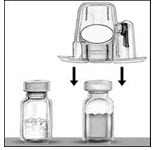
Figure C 
Figure D 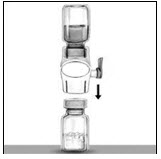
Figure E 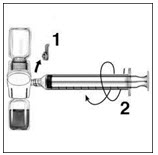
Figure F 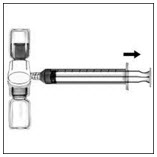
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
RIXUBIS is contraindicated in patients who have:
- Known hypersensitivity to RIXUBIS or its excipients including hamster protein
- Disseminated Intravascular Coagulation (DIC) [see Warnings and Precautions (5.4)]
- Signs of fibrinolysis [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have been reported with RIXUBIS. Anaphylaxis and other hypersensitivity reactions are possible. The risk is highest during the early phases of initial exposure in previously untreated patients (PUPs), in particular in patients with high-risk gene mutations. Early signs of allergic reactions, which can progress to anaphylaxis, include angioedema, chest tightness, hypotension, lethargy, nausea, vomiting, paresthesia, restlessness, wheezing, and dyspnea. Immediately discontinue administration and initiate appropriate treatment if allergic- or anaphylactic-type reactions occur. In case of severe allergic reactions, alternative hemostatic measures should be considered.
There have been reports in the literature showing an association between the occurrence of a factor IX inhibitor and allergic reactions. Evaluate patients experiencing allergic reactions for the presence of an inhibitor.
RIXUBIS contains trace amounts of Chinese hamster ovary (CHO) proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
5.2 Inhibitors
Evaluate patients regularly for the development of factor IX inhibitors by appropriate clinical observations and laboratory tests. Perform an assay that measures factor IX inhibitor concentration if expected factor IX activity plasma levels are not attained, or if bleeding is not controlled with an expected dose. Contact a specialized hemophilia treatment center if a patient develops an inhibitor.
Patients with factor IX inhibitors are at an increased risk of severe hypersensitivity reactions or anaphylaxis if re-exposed to RIXUBIS. RIXUBIS may not be effective in patients with high titer factor IX inhibitors and other therapeutic options should be considered.
5.3 Nephrotic Syndrome
Nephrotic syndrome has been reported following attempted immune tolerance induction in hemophilia B patients with factor IX inhibitors. The safety and efficacy of using RIXUBIS for immune tolerance induction have not been established.
5.4 Thromboembolic Complications
The use of factor IX-containing products has been associated with the development of thromboembolic complications (e.g., pulmonary embolism, venous thrombosis, and arterial thrombosis). Due to the potential risk for thromboembolic complications, monitor patients for early signs of thromboembolic and consumptive coagulopathy, when administering RIXUBIS to patients with liver disease, with signs of fibrinolysis, peri- and post-operatively, or at risk for thromboembolic events or DIC. The benefit of treatment with RIXUBIS should be weighed against the risk of these complications in patients with DIC or those at risk for DIC or thromboembolic events.
5.5 Monitoring Laboratory Tests
- Monitor factor IX activity plasma levels by the one-stage clotting assay to confirm that adequate factor IX levels have been achieved and maintained [see Dosage and Administration (2)].
- Monitor for the development of inhibitors if expected factor IX activity plasma levels are not attained, or if bleeding is not controlled with the recommended dose of RIXUBIS. Assays used to determine if factor IX inhibitor is present should be titered in Bethesda Units (BUs).
-
6 ADVERSE REACTIONS
Common adverse reactions observed in >1% of subjects in clinical studies were dysgeusia, pain in extremity, and positive furin antibody test.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development, in a combined trial, 99 male previously treated patients (PTPs; exposed to a factor IX-containing product for ≥150 days) received at least one infusion of RIXUBIS as part of either on-demand treatment of bleeding episodes, perioperative management of major and minor surgical, dental, or other invasive procedures, routine prophylaxis, or pharmacokinetic evaluation of RIXUBIS. Eleven subjects (11.1%) were <6 years of age, 12 (12.1%) were 6 to <12 years of age, 3 (3%) were adolescents (12 to <16 years of age), and 73 (73.7%) were adults (16 years of age and older). The subjects received a total of 14,018 infusions with a median of 163 infusions of RIXUBIS (range 8 to 327 infusions), for a median of 156 exposure days (range 8 to 316 days).
A total of 337 adverse events were reported in 80 (80.8%) of the 99 subjects. Adverse reactions that occurred in >1% of subjects are shown in Table 3.
Table 3 Summary of Adverse Reactions System Organ Class Adverse Reactions (AR) Number of ARs
(N)Number of Subjects
(N=99)
n (%)Percent per Infusion
(N=14,018)- *
- See Immunogenicity.
Nervous System Disorders Dysgeusia 2 1 (1.01%) 0.014% Musculoskeletal and Connective Tissue Disorders Pain in extremity 1 1 (1.01%) 0.007% Investigations Positive furin antibody test * 2 2 (2.02%) 0.014% Immunogenicity
All 99 subjects were monitored for inhibitory and binding antibodies to factor IX, and binding antibodies to CHO protein and furin, at the following time points: at screening, at 72 hours following the first infusion of RIXUBIS and the commercial recombinant factor IX product in the crossover portion of the pharmacokinetic trial, after 5 and 13 weeks following first exposure to RIXUBIS, and thereafter every 3 months. Antibodies against furin were tested by an in-house enzyme-linked immunosorbent assay (ELISA). A titer of 1:20 or 1:40 was considered to be indeterminate for the above validated assay, as these titers were too low to be verified by the confirmatory assay.
No subjects developed neutralizing antibodies to factor IX. Low-titer, non-neutralizing antibodies against factor IX were observed in 21 (21.2%) subjects at one or more time points. Three of these 21 subjects were found to have these antibodies at screening, prior to receiving RIXUBIS. Six of the 21 subjects were pediatric (2 subjects in <6 years of age cohort, 4 subjects in 6 to <12 years age cohort). No clinical adverse findings were observed in any of these 21 subjects.
Nineteen subjects (19.2%) had signals for antibodies against furin (indeterminate specificity). Five of these 19 subjects expressed signals for antibodies at screening, prior to RIXUBIS treatment. One subject had an antibody signal after treatment with the comparator product and prior to RIXUBIS treatment. Two additional subjects had a positive titer of 1:80 that was not present when checked at a later time point and therefore considered transient. Two of the 19 subjects were pediatric (6 to <12 years age cohort). All post-treatment antibody titer increases in these two pediatric subjects were <2 dilution steps and therefore considered unrelated to treatment. No clinical adverse findings were observed in any of these 19 subjects.
In a trial of 500 normal volunteers, using the same assay as in the clinical trial, 7% had titers of 1:20 or 1:40 and 1.2% had higher titers ranging from 1:80 to 1:320. These antibodies are thought to be part of a natural immune system response. To date, these antibodies have not been associated with any clinical adverse findings.
The detection of antibody formation is dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
Thrombogenicity
There was no clinical evidence of thromboembolic complications in any of the subjects. Out-of-range values for thrombogenicity markers (thrombin-antithrombin III, prothrombin fragment 1.2, and D-dimer), determined during the pharmacokinetic portion of the combined trial, did not reveal any pattern indicative of clinically relevant thrombogenicity with either RIXUBIS or a comparator factor IX-containing product.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of RIXUBIS. Because the following reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity (including symptoms such as dyspnea, pruritus)
Skin and Subcutaneous Tissue Disorders: Urticaria, rash
The following class adverse reactions have been seen with another recombinant factor IX: inadequate factor IX recovery, inhibitor development, anaphylaxis, angioedema, hypotension, and thrombosis.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with RIXUBIS use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with RIXUBIS. It is also not known whether RIXUBIS can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively, regardless of drug exposure.
8.2 Lactation
Risk Summary
There is no information regarding the presence of RIXUBIS in human milk, the effect on the breastfed infant, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for RIXUBIS and any potential adverse effects on the breastfed child from RIXUBIS or from the underlying maternal condition.
8.4 Pediatric Use
Safety, efficacy and pharmacokinetics of RIXUBIS have been evaluated in 23 previously treated pediatric patients. Twelve (52.2%) were 6 to <12 years of age and 11 subjects (47.8%) were <6 years of age. Previously treated pediatric subjects 6 to <12 years of age were previously treated with plasma-derived and/or recombinant factor IX concentrate(s) for a minimum of 150 exposure days (based on the subject's medical records). Subjects <6 years of age were previously treated with plasma-derived and/or recombinant factor IX concentrate(s) for > 50 exposure days (based on the subject's medical records).
Incremental recovery was observed to be 22% lower in pediatric patients (<12 years) and dose adjustment is needed [see Dosage and Administration (2) and Clinical Pharmacology (12.3)]. The mean incremental recovery (in [IU/dL]/[IU/kg]) at the initial pharmacokinetics evaluation was 0.67 (± 0.16) in subjects of both age cohorts, with lower values in the younger age cohort (0.59 ± 0.13) and higher values in the older age group (0.73 ± 0.16). Clearance was higher in the younger age cohort, with a mean clearance of 10.6 ± 1.7 mL/(kg.hr) (median: 10.5; range: 8.1-14.4) in the <6 years age cohort compared to a mean clearance of 8.7 ± 1.2 mL/(kg.hr) (median: 8.6; range: 6.9-10.8) in the 6 to <12 years age cohort.
In a prospective, open-label, uncontrolled multicenter trial with patients <12 years of age, a total of 41 infusions were given for the treatment of 26 bleeding episodes (7 joint, 19 muscle and soft tissue). Bleeding was controlled in all episodes. In 23 of 26 (88.5%) of the episodes, hemostasis was achieved with one or two infusions. The mean annualized bleeding episode rate (ABR) for children <12 years of age was 2.7 bleeds per patient per year ± 3.14 with a median of 2 ranging from 0 to 10.8. See Clinical Studies (14) for patients 12 to <16 years of age. The median ABR during prophylaxis was comparable among children, adolescents and adults.
8.5 Geriatric Use
Clinical studies of RIXUBIS did not include subjects aged 65 and over. It is not known whether elderly patients respond differently than younger patients. Dose selection for an elderly patient should be individualized [see Dosage and Administration (2)].
-
11 DESCRIPTION
RIXUBIS [Coagulation Factor IX (Recombinant)] is a purified protein produced by recombinant DNA technology. Its amino acid sequence is identical to that of the Ala-148 allelic form of plasma derived factor IX, and its structural and functional characteristics are similar to those of plasma derived factor IX. RIXUBIS is produced by a genetically engineered CHO cell line. No human or animal proteins are added during any stage of manufacturing or formulation of RIXUBIS. The CHO cell line secretes recombinant factor IX into a defined cell culture medium that does not contain hormones, and the recombinant factor IX is purified by a chromatography purification process that does not require a monoclonal antibody step. The process includes validated virus inactivation/removal steps, namely solvent/detergent treatment and 15 nm nanofiltration. RIXUBIS is predominantly a single component by sodium dodecyl sulfate-polyacrylamide gel electrophoresis evaluation. The specific activity of RIXUBIS is ≥200 international units per milligram of protein. Factor IX preactivation, the percent of Factor IXa/Factor IX as measured by activity assays, is ≤0.03%. The potency in international units is determined using an in vitro thromboplastin time (aPTT)-based one-stage clotting assay calibrated against the World Health Organization (WHO) International Standard for Factor IX concentrate. Factor IX potency results can be affected by the type of aPTT reagent and reference standard used in the assay; differences of up to 40% have been observed.
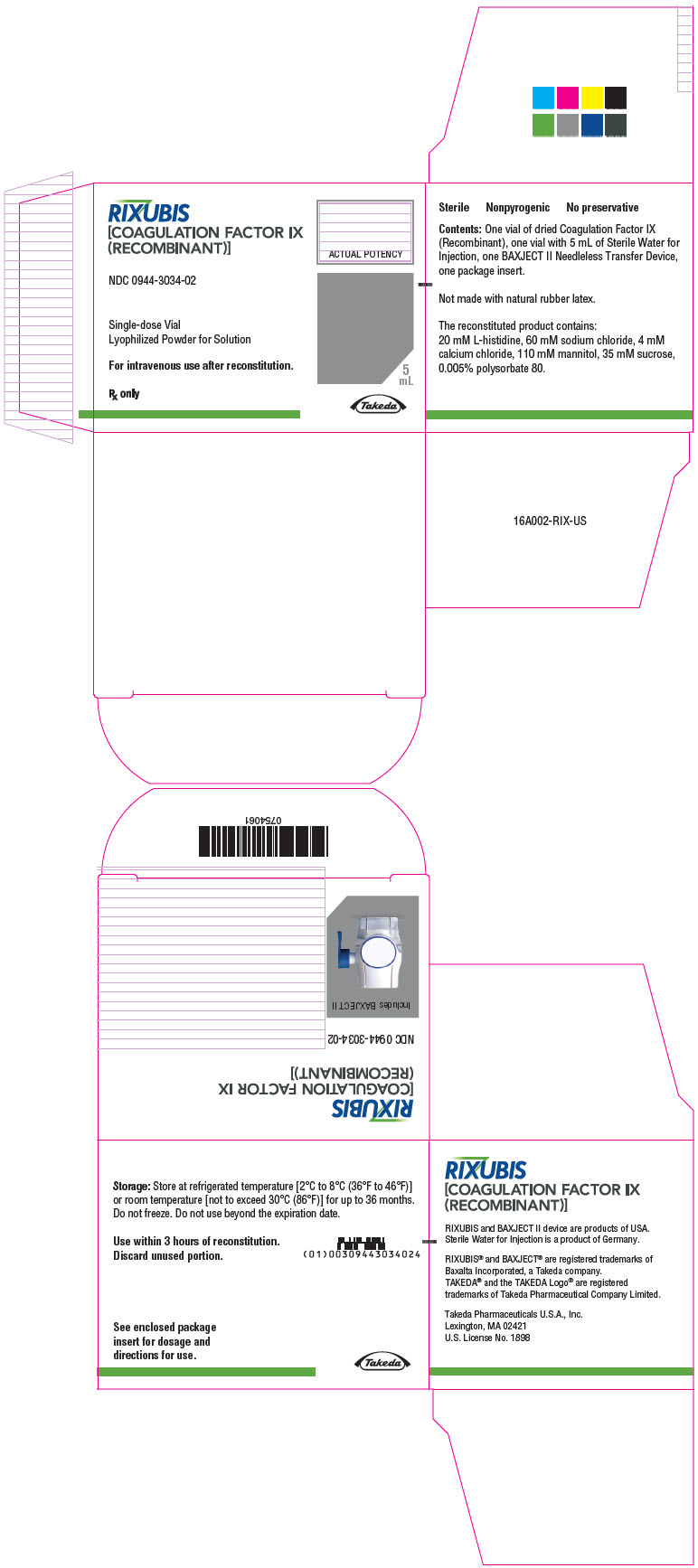
RIXUBIS is formulated as a sterile, nonpyrogenic lyophilized powder to be reconstituted with Sterile Water for Injection for intravenous administration. It does not contain any preservatives and is available in single-dose vials containing the labeled amount of factor IX activity, expressed in international units. Each vial contains nominally 250, 500, 1000, 2000 or 3000 international units of recombinant coagulation factor IX. After reconstitution of the lyophilized powder, all dosage strengths yield a clear, colorless solution. The concentrations of excipients are shown in Table 4:
Table 4 Concentrations of excipients Excipient Concentration L-histidine 20 mM Sodium chloride 60 mM Calcium chloride 4 mM Mannitol 110 mM Sucrose 35 mM Polysorbate 80 0.005% -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Patients with hemophilia B are deficient in coagulation factor IX, which is required for effective hemostasis. Treatment with RIXUBIS temporarily replaces the missing coagulation factor IX.
12.2 Pharmacodynamics
The administration of RIXUBIS increases plasma levels of factor IX and can temporarily correct the coagulation defect in these patients by decreasing the aPTT.
12.3 Pharmacokinetics
PTPs ≥12 Years of Age
A randomized, blinded, controlled pharmacokinetic trial of RIXUBIS was conducted in non-bleeding subjects (≥15 years of age). The subjects received RIXUBIS as an intravenous infusion. The infusion rate for the first administration during the trial was not to exceed 4 mL/minute with a maximum infusion rate of 10 mL/minute. The dose range of RIXUBIS was from 71.3 to 79.4 international units/kg (mean dose = 74.7 international units/kg). Blood samples for factor IX activity measurements were obtained up to 72 hours and a non-compartmental analysis was used to estimate pharmacokinetic parameters.
The pharmacokinetic evaluation was repeated for RIXUBIS in a prospective, open-label, uncontrolled trial with RIXUBIS in subjects who participated in the initial trial and had then received RIXUBIS for 26 ± 1 (mean ± SD) weeks for routine prophylaxis and accumulated at least 30 exposure days to RIXUBIS. The dose range of RIXUBIS in this repeat pharmacokinetic trial was 64.5 to 79.2 international units/kg (mean dose = 74.6 international units/kg). Table 5 presents the pharmacokinetic parameters for all evaluable subjects (per protocol analysis).
Table 5 Pharmacokinetic Parameters for RIXUBIS Following Single and Repeat Dosing (≥12 years of age) Parameter First Dose
(N=25)Repeat Dose
(N=23)AUC0-inf (IU∙hrs/dL) * Mean (SD) 1207 (242) 1305 (300) Min; Max 850; 1710 838; 1864 Incremental recovery at Cmax (IU/dL:IU/kg) † Mean (SD) 0.87 (0.22) 0.95 (0.25) Min; Max 0.53; 1.35 0.52; 1.38 Half-life (hrs) Mean (SD) 26.7 (9.6) 25.4 (6.9) Min; Max 15.8; 52.3 16.2; 42.2 Cmax (IU/dL) Mean (SD) 66.2 (15.8) 72.7 (19.7) Min; Max 41.7; 100.3 38.5; 106.3 Mean Residence Time (hrs) Mean (SD) 30.8 (7.3) 29.9 (4.2) Min; Max 22.3; 47.8 21.3; 37.5 VSS‡(mL/kg) Mean (SD) 201.9 (77.4) 178.6 (45.2) Min; Max 110.0; 394.0 112.0; 272.0 Clearance [mL/(kg∙hr)] Mean (SD) 6.4 (1.3) 6.0 (1.5) Min; Max 4.3; 9.1 4.1; 9.5 Data from PTPs who underwent repeat in vivo recovery testing for up to 26 weeks demonstrated that the incremental factor IX recovery was consistent over time (Table 6).
Table 6 Incremental Recovery for RIXUBIS 30 Minutes After Infusion (≥12 years of age) Incremental recovery 30 min after infusion (IU/dL:IU/kg) * Day 1
(N=73)Week 5
(N=71)Week 13
(N=68)Week 26
(N=55)At trial completion/termination†
(N=23)Mean ± SD 0.79 ± 0.20 0.83 ± 0.21 0.85 ± 0.25 0.89 ± 0.12 0.87 ± 0.20 Median (range) 0.78 (0.26-1.35) 0.79 (0.46-1.48) 0.83 (0.14-1.47) 0.88 (0.52-1.29) 0.89 (0.52-1.32) PTPs <12 Years of Age
Twenty-three male subjects underwent a pharmacokinetic evaluation of RIXUBIS as part of the combined pediatric trial (<6 years and 6 -<12 years). The mean (± SD) and median dose were 75.5 ± 3.0 and 75.3 international units/kg, respectively, with a range of 70.0 to 83.6 international units/kg. Non-linear mixed model (population PK) was used to estimate the pharmacokinetic parameters from factor IX activity measurements in blood samples obtained up to 60 hours following the infusion. Pharmacokinetic parameters for all subjects are presented in Table 7.
Table 7 Pharmacokinetic Parameters for PTPs Parameter ≥12 years*
(N=25)6 - <12 years
(N=12)<6 years
(N=11)- *
- Non-linear mixed model (population PK) was applied on the reduced 4 blood samples (30min, 6hr, 24hr, and 60hr).
AUC0–inf (IU∙hr/dL) * Mean ± SD 1185 ± 273 886 ± 134 724 ± 119 Median (range) 1197 (783-1780) 864 (730-1138) 717 (488-947) Half-life (hr) Mean ± SD 25.7 ± 1.5 23.2 ± 1.6 27.7 ± 2.7 Median (range) 25.6 (22.8-29.0) 22.6 (21.8-27.4) 27.3 (24.0-32.2) Mean residence time (hr) Mean ± SD 30.2 ± 2.2 25.3 ± 1.8 30.6 ± 3.3 Median (range) 30.3 (25.9-33.9) 24.7 (23.7-30.3) 30.1 (26.2-36.2) VSSb (mL/kg) Mean ± SD 201.5 ± 47.2 220.9 ± 31.7 322.5 ± 52.3 Median (range) 190.2 (138-300) 218.5 (169.9-270.1) 315.7 (264.7-441.5) Clearance (mL/[kg∙hr]) Mean ± SD 6.7 ± 1.5 8.7 ± 1.2 10.6 ± 1.7 Median (range) 6.43 (4.1-9.9) 8.6 (6.9-10.8) 10.5 (8.1-14.4) Incremental recovery 30 minutes after infusion was determined for all subjects in the combined trial during the pharmacokinetic evaluation (exposure day 1), at week 5, 13, and 26 visits, and at the time of trial completion or termination, if it did not coincide with the week 26 visit. The data demonstrate that the incremental recovery is consistent over time across all pediatric age groups. (see Tables 8 and 9)
Table 8 Incremental Recovery for RIXUBIS 30 Minutes After Infusion Ages < 6 Years Incremental recovery 30 min after infusion PK (ED 1)
(N=10)Week 5
(N=11)Week 13
(N=10)Week 26
(N=10)- *
- Calculated as (C30min–baseline factor IX) divided by the dose in IU/kg, where C30min is the factor IX measurement 30 minutes after infusion.
(IU/dL÷IU/kg) * Mean ± SD 0.59 ± 0.13 0.63 ± 0.10 0.68 ± 0.12 0.65 ± 0.13 Median (range) 0.59 (0.31-0.75) 0.6 (0.49-0.80) 0.66 (0.51-0.84) 0.61 (0.51-0.84) Table 9 Incremental Recovery for RIXUBIS 30 Minutes After Infusion Ages 6 - <12 Years Incremental recovery 30 min after infusion PK (ED 1)
N=12)Week 5
(N=12)Week 13
(N=11)Week 26
(N=11)- *
- Calculated as (C30min–baseline factor IX) divided by the dose in IU/kg, where C30min is the factor IX measurement 30 minutes after infusion.
(IU/dL÷IU/kg) * Mean ± SD 0.73 ± 0.16 0.73 ± 0.13 0.73 ± 0.14 0.8 ± 0.14 Median (range) 0.71 (0.51 – 1.00) 0.70 (0.48-0.92) 0.70 (0.54-1.00) 0.78 (0.56-1.01) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Nonclinical studies evaluating the carcinogenic and mutagenic potential of RIXUBIS have not been conducted.
No adverse effects on reproductive organs were observed by macroscopic and microscopic pathological investigations in repeated dose toxicity studies. No investigations on impairment of fertility have been conducted.
-
14 CLINICAL STUDIES
On-demand Treatment of Bleeding Episodes in PTPs ≥12 Years of Age
A total of 249 bleeding episodes were treated with RIXUBIS, of which 115 bleeds were recorded for subjects treated on prophylaxis and 134 bleeds for those with the on-demand regimen. There were 197 joint bleeds and 52 non-joint bleeds (soft tissue, muscle, body cavity and other). Of the 249 bleeding episodes, 15 were major, 163 were moderate, and 71 were minor (see Table 1 for definitions of major, moderate and minor). Treatment was individualized based on the severity, cause, and site of bleed. The majority (211; 84.7%) were treated with 1 to 2 infusions. Of these, 153 (61.4%) were treated with 1 infusion and 58 (23.3%) were treated with 2 infusions. No non-responders were reported.
Hemostatic efficacy at resolution of a bleed was rated excellent or good in 96% of all treated bleeding episodes. (Excellent is defined as full relief of pain and cessation of objective signs of bleeding after a single infusion; no additional infusion is required for the control of bleeding; Good is defined as definite pain relief and/or improvement in signs of bleeding after a single infusion; possibly requires more than one infusion for complete resolution).
On-demand Treatment of Bleeding Episodes in PTPs <12 Years of Age
A total of 26 bleeding episodes were treated with RIXUBIS, of which 23 bleeds were due to injury, 2 spontaneous and 1 of unknown origin; 19 bleeds were non-joint (soft tissue, muscle, body cavity, intracranial and other) and 7 were joint bleeds of which 1 was a bleed into a target joint. Of the 26 bleeding episodes, 15 were minor, 9 moderate, and 2 major (Minor: little or no pain; little or no change in the range of motion of affected joint (if joint bleeding event); mild restriction of mobility and activity; Moderate: mild to moderate pain; some decrease in range of motion of affected joint (if joint bleeding event); moderate decrease in mobility and activity; Major/life threatening: significant pain; substantial decrease in range of motion of affected joint (if joint bleeding event); incapacity; life threatening). Treatment was individualized based on the severity, cause and site of bleed. The majority (23; 88.5%) were treated with 1 to 2 infusions. Of the treated bleeding episodes 15 (57.7%) received 1 infusion, 8 (30.8%) received 2 infusions, and 3 (11.5%) were treated with 3 infusions. Hemostatic efficacy at resolution of a bleed was rated excellent or good in 96.2% of all treated bleeding episodes.
Perioperative Management
The efficacy analysis of RIXUBIS in perioperative management included 14 surgeries performed in 14 PTPs between 19 and 54 years of age undergoing major or minor surgical (see Table 2 for definitions of major and minor), dental or other surgical invasive procedures. Eleven procedures (including 7 orthopedic and 1 dental surgeries) were major, 3 procedures (including 2 dental extractions) were minor.
Subjects undergoing major surgeries also underwent a pharmacokinetic evaluation. All subjects were dosed based on their most recent individual incremental recovery. The recommended initial loading dose of RIXUBIS was used to ensure that during surgery factor IX activity levels of 80-100% for major surgeries and 30-60% for minor surgeries were maintained. RIXUBIS was administered by intravenous bolus infusions. Hemostasis was maintained throughout the trial duration. The following are the surgical procedures and results of the assessment of the hemostatic response at various points.
Fourteen surgeries (11 major; 3 minor), included in the intraoperative assessment had a rating of 'excellent'. At discharge from hospital 11/14 surgeries (8 major) had a rating of 'excellent' and 3/14 (3 major) had a rating of 'good'.
The rating of excellent, good, fair and none at 72 hours post-surgery was based on the hemostasis achieved as compared to that expected in hemostatically normal subjects.
Routine Prophylaxis and Control of Bleeding in PTPs ≥12 Years of Age
The efficacy of RIXUBIS has been evaluated in a prospective, open-label, uncontrolled multicenter trial, in which a total of 73 male PTPs between 12 and 65 years of age received RIXUBIS either for routine prophylaxis or on-demand treatment. In addition, there is an ongoing prospective, open-label, uncontrolled, multicenter trial where 14 PTPs underwent minor or major surgeries receiving RIXUBIS for perioperative management. PTPs were defined as subjects who were exposed to a factor IX containing product for ≥150 days. All subjects had severe (factor IX level <1%) or moderately severe (factor IX level ≤2%) hemophilia B. The majority of subjects (88%) had arthropathy and/or target joints (66%) at screening. Subjects with history of a detectable factor IX inhibitor ≥0.6 BU, history of severe allergic reactions following exposure to factor IX containing products, evidence of severe chronic liver disease (INR >1.4), impaired renal function, CD4 count <200 cells/mm3, or any hemostatic defect other than hemophilia B, were excluded from the trial.
Of 59 subjects who received RIXUBIS for routine prophylaxis, 56 subjects received the product for a minimum of 3 months and were included in the efficacy evaluation. The prophylactic regimen consisted of 40 to 60 international units/kg RIXUBIS twice weekly. An additional 14 subjects received RIXUBIS on-demand for treatment of bleeding episodes only. These subjects had to have at least 12 documented bleeding episodes requiring treatment within 12 months prior to enrollment.
In the on-demand arm, the mean treatment duration was 3.5 ± 1 months (median 3.4, ranging from 1.2 to 5.1 months) and the mean total annualized bleeding rate (ABR was 33.9 ± 17.37 with a median of 27 ranging from 12.9 to 73.1).
In the prophylaxis arm the mean ABR was 4.3 for all bleeds, 1.7 for spontaneous bleeds, and 2.9 for joint bleeds (Table 10).
Table 10 Efficacy of Routine Prophylaxis with RIXUBIS (N=56) ≥12 Years of Age *The prophylactic regimen consisted of 40 to 60 international units/kg RIXUBIS twice weekly. Total ABR Mean ± SD 4.3 ± 5.80 Median (range) 2.0 (0.0-23.4) ABR for joint bleeds Mean ± SD 2.9 ± 4.25 Median (range) 0.0 (0.0-21.5) ABR for spontaneous bleeds Mean ± SD 1.7 ± 3.26 Median (range) 0.0 (0.0-15.6) Subjects with zero bleeding episodes % (n) 42.9% (24) Routine Prophylaxis and Control of Bleeding in PTPs <12 Years of Age
The efficacy of RIXUBIS has been evaluated in a clinical trial, in which a total of 23 male, (PTPs) between 1.8 and 11.8 years (median age 7.10 years) with 11 subjects <6 years, received RIXUBIS for prophylaxis and control of bleeding episodes. PTPs were defined as subjects who were exposed to a factor IX-containing product on ≥150 days for subjects aged 6 to <12 years and on ≥50 days for subjects aged <6 years. All subjects had severe (factor IX level <1%) or moderately severe (factor IX level ≤2%) hemophilia B. Subjects with a history of or a detectable FIX inhibitor ≥0.6 BU, a history of severe allergic reactions following exposure to FIX, evidence of severe chronic liver disease (INR >1.4), impaired renal function, a CD4 count <200 cells/mm3 or any hemostatic effect other than hemophilia B were excluded from participation. All 23 subjects received prophylactic treatment with RIXUBIS for a minimum of 3 months and were included in the efficacy evaluation for prophylaxis (see Table 11).
Table 11 Efficacy of Routine Prophylaxis of RIXUBIS in 23 PTPs <12 Years of Age * The prophylactic regimen consisted of 40 to 80 international units/kg RIXUBIS twice weekly. Total annualized bleeding rate (ABR) Mean ± SD 2.7 ± 3.14 Median (range) 2.0 (0.0–10.8) ABR for joint bleeds Mean ± SD 0.8 ± 1.76 Median (range) 0.0 (0.0–7.2) ABR for spontaneous bleeds Mean ± SD 0.2 ± 0.66 Median (range) 0.0 (0.0–2.0) Subjects with zero bleeds % (n) 39.1.% (9) - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
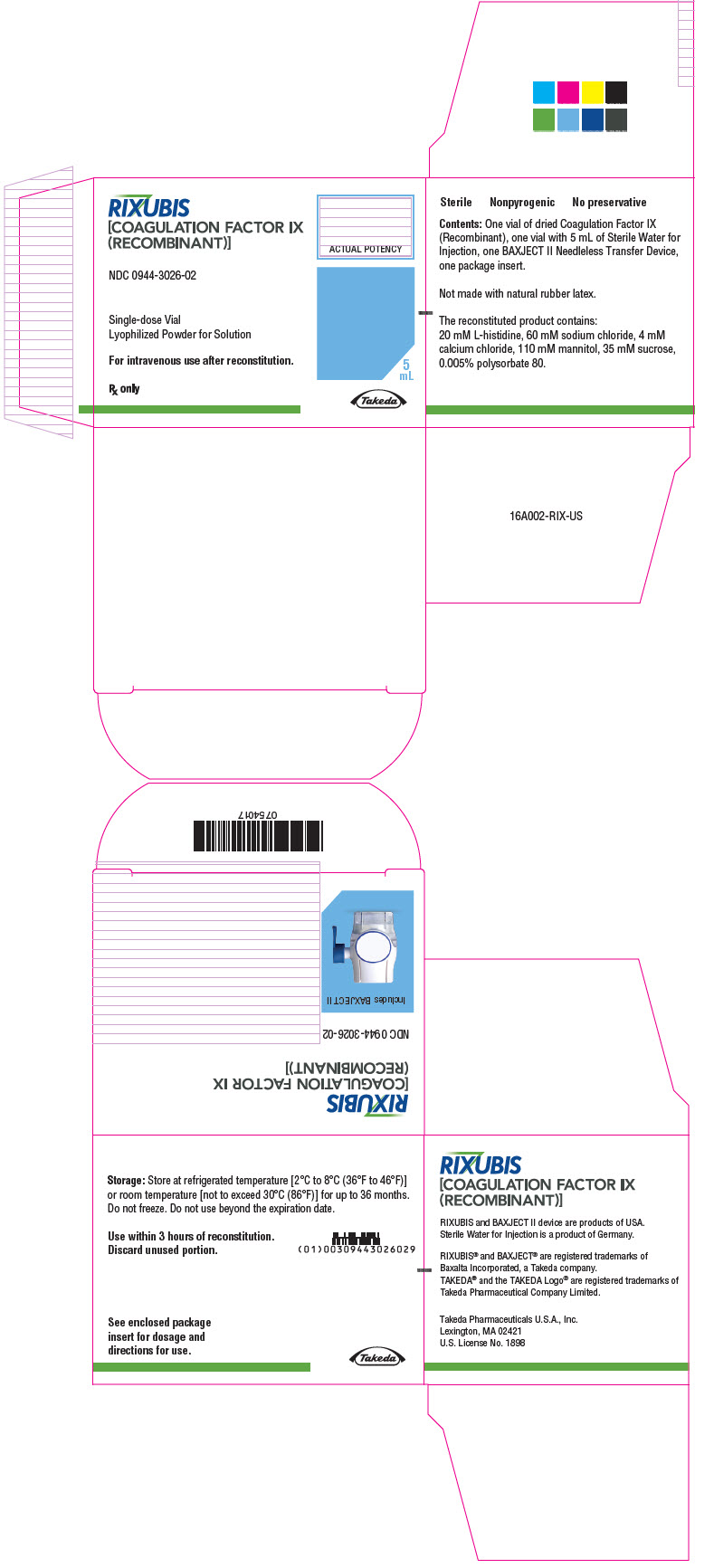
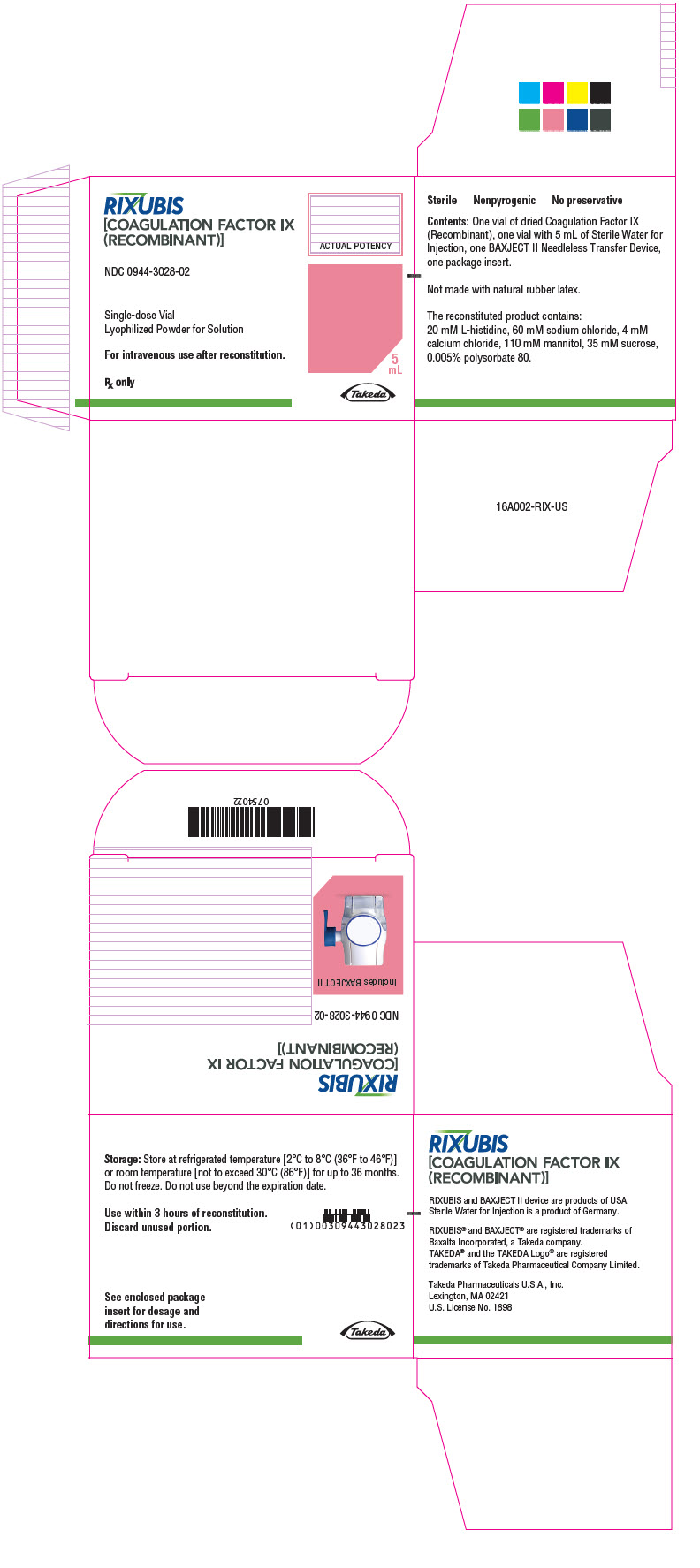
RIXUBIS is available as single-dose vials containing the following product strengths:
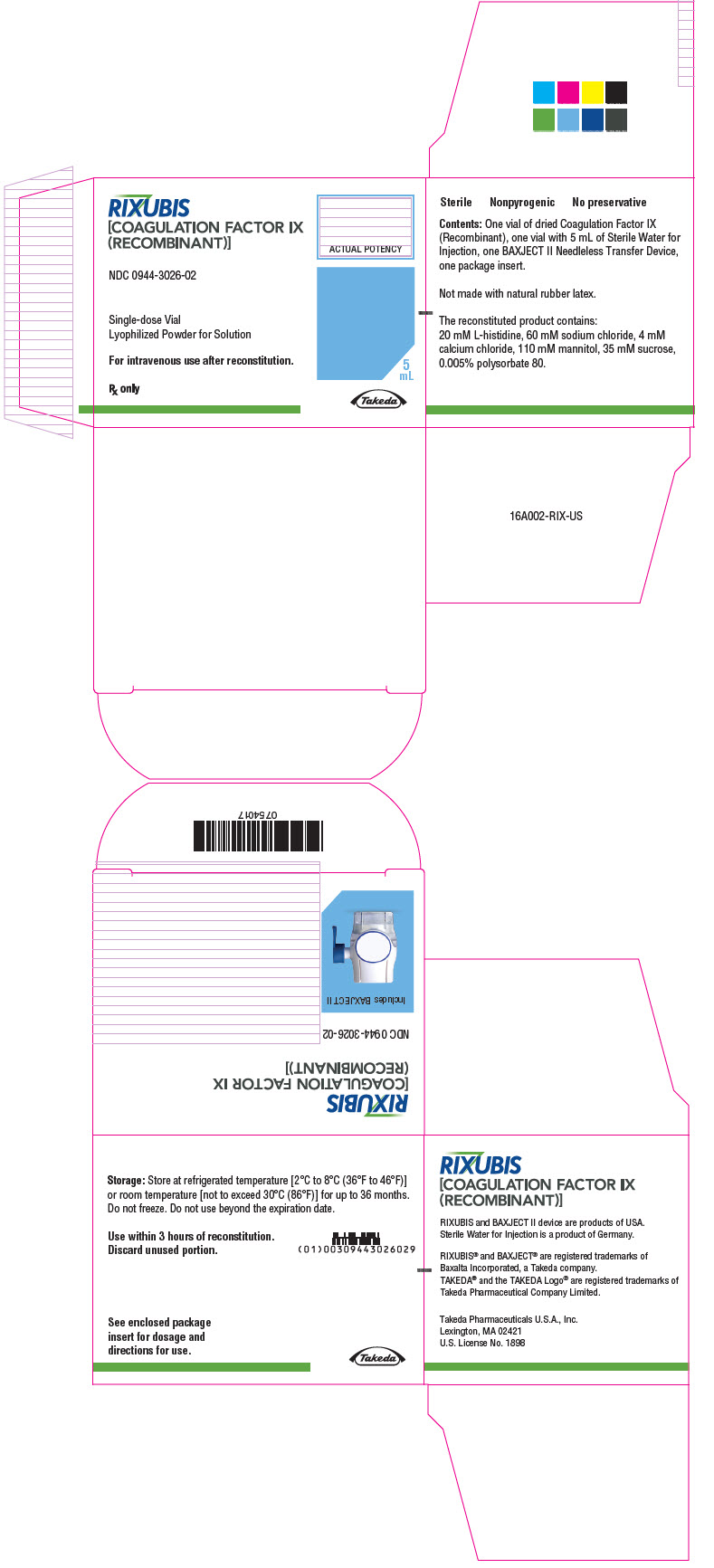
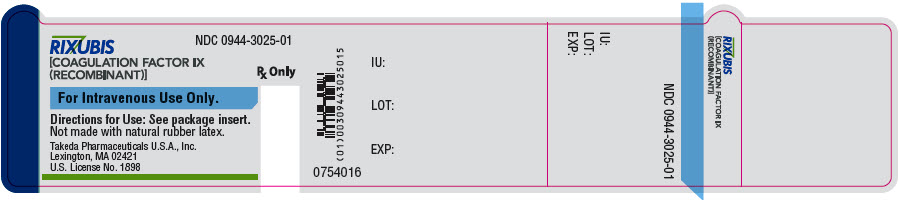
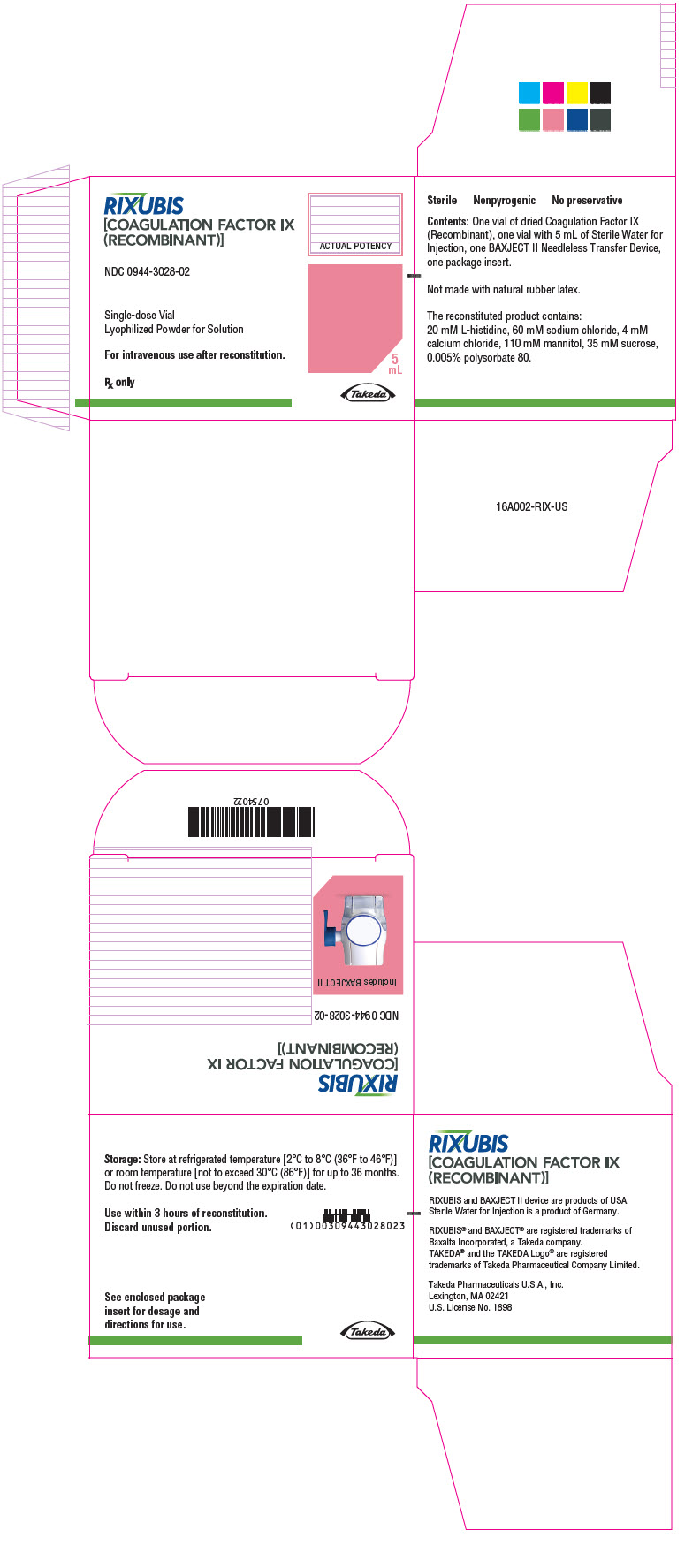
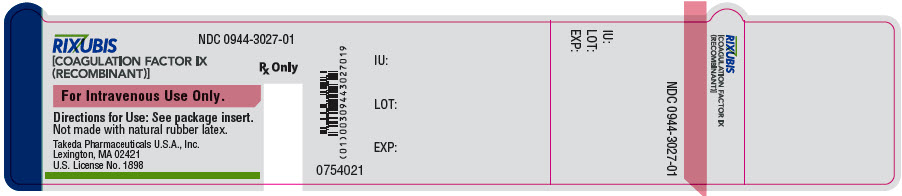
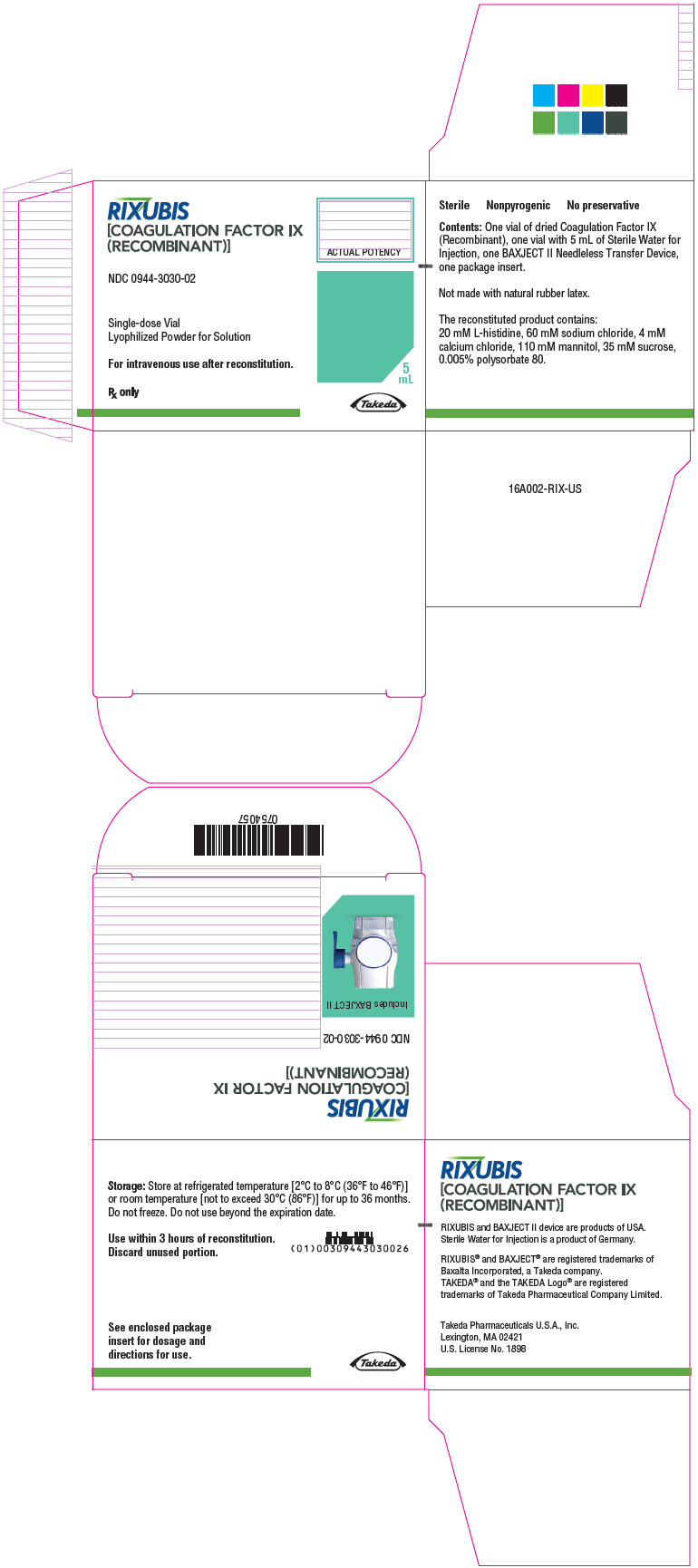
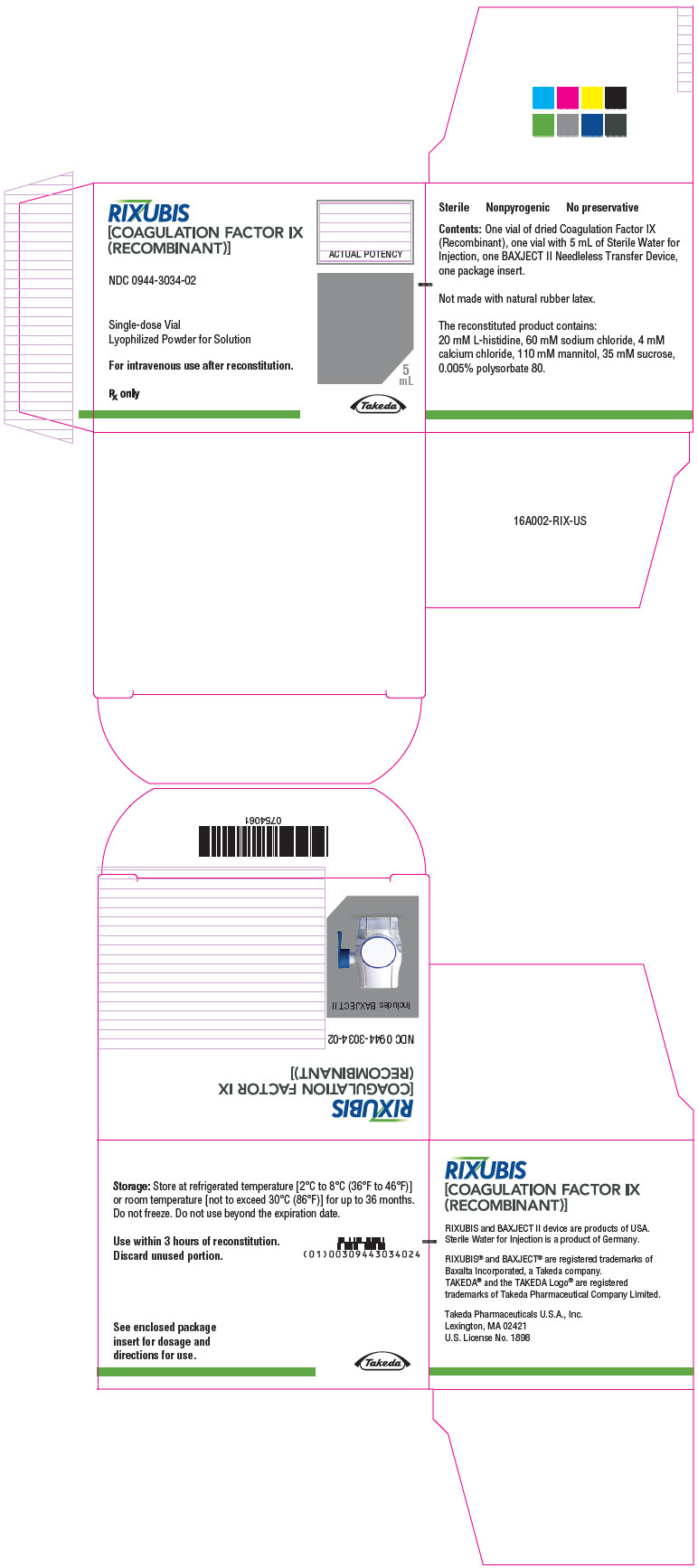
Color Code Nominal Strength (international units) Kit NDC Light Blue 250 0944-3026-02 Pink 500 0944-3028-02 Green 1000 0944-3030-02 Orange 2000 0944-3032-02 Silver 3000 0944-3034-02 Actual factor IX activity in international units is stated on the unit carton and vial label.
Each kit also contains 5 mL of Sterile Water for Injection and a BAXJECT II transfer device.
Not made with natural rubber latex.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Advise patients to report any adverse reactions or problems following RIXUBIS administration to their physician or healthcare provider.
- Inform patients of the early signs of hypersensitivity reactions (including hives, generalized urticaria, chest tightness, wheezing, and hypotension) and anaphylaxis. Instruct patients to discontinue use of the product and contact their physician if these symptoms occur.
- Advise patients to contact their physician or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to factor IX replacement therapy, as in some cases this may be a manifestation of an inhibitor.
- Ask patients to follow the specific preparation and administration procedures provided by their physician.
- Inform patients to follow the recommendations in the FDA-approved patient labeling.
- SPL UNCLASSIFIED SECTION
-
Patient Information
RIXUBIS
[Coagulation Factor IX (Recombinant)]This leaflet summarizes important information about RIXUBIS. Please read it carefully before using this medicine. This information does not take the place of talking with your healthcare provider, and it does not include all of the important information about RIXUBIS. If you have any questions after reading this, ask your healthcare provider.
What is RIXUBIS?
RIXUBIS is a medicine used to replace clotting factor (Factor IX) that is missing in people with hemophilia B. Hemophilia B is also called congenital factor IX deficiency or Christmas disease. Hemophilia B is an inherited bleeding disorder that prevents blood from clotting normally. RIXUBIS is used to prevent and control bleeding in people with hemophilia B. Your healthcare provider may give you RIXUBIS when you have surgery. RIXUBIS can reduce the number of bleeding episodes when used regularly (prophylaxis).
Who should not use RIXUBIS?
You should not use RIXUBIS if you
- are allergic to hamsters
- are allergic to any ingredients in RIXUBIS.
Tell your healthcare provider if you are pregnant or breastfeeding because RIXUBIS may not be right for you.
What should I tell my healthcare provider before using RIXUBIS?
You should tell your healthcare provider if you
- have or have had any medical problems
- take any medicines, including prescription and non-prescription medicines, such as over-the-counter medicines, supplements or herbal remedies
- have any allergies, including allergies to hamsters
- are breastfeeding. It is not known if RIXUBIS passes into your milk and if it can harm your baby
- are pregnant or planning to become pregnant. It is not known if RIXUBIS may harm your unborn baby
- have been told that you have inhibitors to factor IX (because RIXUBIS may not work for you).
How should I infuse RIXUBIS?
RIXUBIS is given directly into the bloodstream. RIXUBIS should be administered as ordered by your healthcare provider. You should be trained on how to do infusions by your healthcare provider or hemophilia treatment center. Many people with hemophilia B learn to infuse their RIXUBIS by themselves or with the help of a family member.
Your healthcare provider will tell you how much RIXUBIS to use based on your weight, the severity of your hemophilia B, and where you are bleeding. You may have to have blood tests done after getting RIXUBIS to be sure that your blood level of factor IX is high enough to clot your blood. Call your healthcare provider right away if your bleeding does not stop after taking RIXUBIS.
What are the possible side effects of RIXUBIS?
Allergic reactions may occur with RIXUBIS. Call your healthcare provider or get emergency treatment right away if you get a rash or hives, itching, tightness of the throat, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea or fainting. Some common side effects of RIXUBIS were unusual taste in the mouth and limb pain. Tell your healthcare provider about any side effects that bother you or do not go away. These are not all the side effects possible with RIXUBIS. You can ask your healthcare provider for information that is written for healthcare professionals.
Your body may form inhibitors to factor IX. An inhibitor is part of the body's defense system. If you form inhibitors, it may stop RIXUBIS from working properly. Consult with your healthcare provider to make sure you are carefully monitored with blood tests for the development of inhibitors to factor IX.
What are the RIXUBIS dosage strengths?
RIXUBIS comes in five different dosage strengths: 250, 500, 1000, 2000 and 3000 international units. The actual strength will be imprinted on the label and on the box. The five different strengths are color coded, as follows:

Dosage strength of approximately 250 international units per vial 
Dosage strength of approximately 500 international units per vial 
Dosage strength of approximately 1000 international units per vial 
Dosage strength of approximately 2000 international units per vial 
Dosage strength of approximately 3000 international units per vial Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store RIXUBIS?
- Store at refrigerated temperature [2°C to 8°C (36°F to 46°F)] or room temperature [not to exceed 30°C (86°F)] for up to 36 months. Do not freeze.
- Do not use after the expiration date printed on the carton or vial.
- Reconstituted product (after mixing dry product with wet diluent) must be used within 3 hours and cannot be stored or refrigerated. Discard any RIXUBIS left in the vial at the end of your infusion.
What else should I know about RIXUBIS?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use RIXUBIS for a condition for which it is not prescribed. Do not share RIXUBIS with other people, even if they have the same symptoms that you have.
Resources available to patients
For information on patient assistance programs that are available to you, please contact 1-888-229-8379.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898RIXUBIS® and BAXJECT® are registered trademarks of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited. -
INSTRUCTIONS FOR USE
RIXUBIS
[Coagulation Factor IX (Recombinant)]
For intravenous use only
Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia center.
Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using RIXUBIS. If you are unsure of the procedures, please call your healthcare provider before using RIXUBIS. Call your healthcare provider right away if bleeding is not controlled after using RIXUBIS. Your healthcare provider will prescribe the dose that you should take. Your healthcare provider may need to take blood tests from time to time. Talk to your healthcare provider before traveling. Plan to bring enough RIXUBIS for your treatment during this time. Dispose of all materials, including any leftover reconstituted RIXUBIS product, in an appropriate container.
- Prepare a clean flat surface and gather all the materials you will need for the infusion. Check the expiration date, and let the vial with the RIXUBIS concentrate and the Sterile Water for Injection, USP (diluent) warm up to room temperature. Wash your hands and put on clean exam gloves. If infusing yourself at home, the use of gloves is optional.
- Remove caps from the RIXUBIS concentrate and diluent vials to expose the centers of the rubber stoppers.
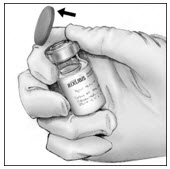
- Disinfect the stoppers with an alcohol swab (or other suitable solution suggested by your healthcare provider or hemophilia center) by rubbing the stoppers firmly for several seconds and allow them to dry prior to use. Place the vials on a flat surface.
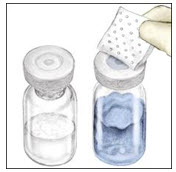
- Open the BAXJECT II device package by peeling away the lid, without touching the inside of the package. Do not remove the BAXJECT II device from the package.
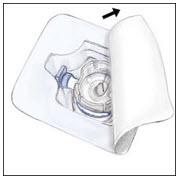
- Turn the package with the BAXJECT II device upside down and place it over the top of the diluent vial. Fully insert the clear plastic spike of the device into the center of the diluent vial stopper by pushing straight down. Grip the package at its edge and lift it off the device. Be careful not to touch the white plastic spike. Do not remove the blue cap from the BAXJECT II device. The diluent vial now has the BAXJECT II device connected to it and is ready to be connected to the RIXUBIS vial.
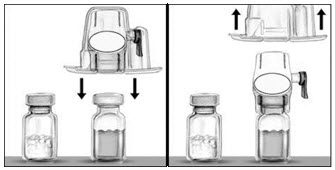
- To connect the diluent vial to the RIXUBIS vial, turn the diluent vial over and place it on top of the vial containing RIXUBIS concentrate. Fully insert the white plastic spike into the RIXUBIS vial stopper by pushing straight down. Diluent will flow into the RIXUBIS vial. This should be done right away to keep the liquid free of germs.

- Swirl the connected vials gently and continuously until the powder is completely dissolved. Do not shake. The RIXUBIS solution should look clear and colorless. If not, do not use it and notify Takeda Pharmaceuticals immediately.

- Take off the blue cap from the BAXJECT II device and connect the syringe by screwing it clockwise until the syringe is secured. Do not over tighten. Be careful to not inject air.
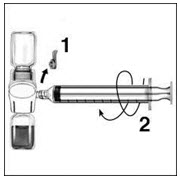
- Turn over the connected vials so that the RIXUBIS vial is on top. Draw the RIXUBIS solution into the syringe by pulling back the plunger slowly. Disconnect the syringe from the BAXJECT II unscrewing it counterclockwise.
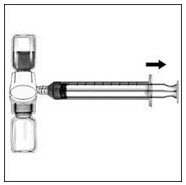
- If you are using more than one vial of RIXUBIS, the contents of more than one vial may be drawn into the same syringe. Make sure you mix each vial of RIXUBIS with the Sterile Water for Injection, USP that is provided in the box (following Steps 1-9). You will need a separate BAXJECT II device to mix each additional vial of RIXUBIS.
- Attach the infusion needle to the syringe using a winged (butterfly) infusion set, if available. Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
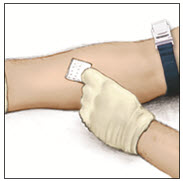
- Apply a tourniquet and get the infusion site ready by wiping the skin well with an alcohol swab (or other suitable solution suggested by your healthcare provider or hemophilia center).
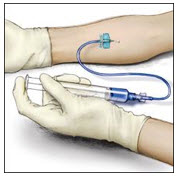
- Insert the needle into the vein and remove the tourniquet. Slowly infuse the RIXUBIS. Do not infuse any faster than 10 mL per minute.
- Take the needle out of the vein and use sterile gauze to put pressure on the infusion site for several minutes. Do not recap the needle. Place it with the used syringe in a hard-walled sharps container for proper disposal.
- Dispose of the used vials and BAXJECT II system in your hard-walled sharps container without taking them apart. Do not dispose of these supplies in ordinary household trash.
- Remove the peel-off label from the RIXUBIS vial and place it in your logbook. Clean any spilled blood with a freshly prepared mixture of 1 part bleach and 9 parts water, soap and water, or any household disinfecting solution.
Important: Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898RIXUBIS® and BAXJECT® are registered trademarks of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.Issue 3/2023
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 0944-3026-02
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 0944-3025-01
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 0944-3028-02
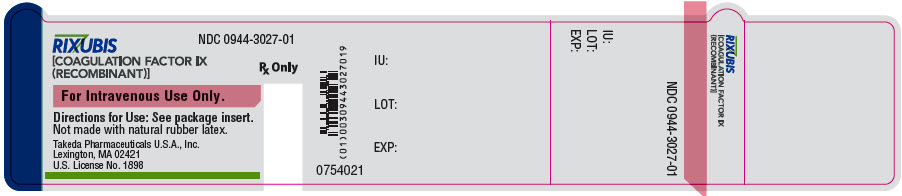
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 0944-3027-01
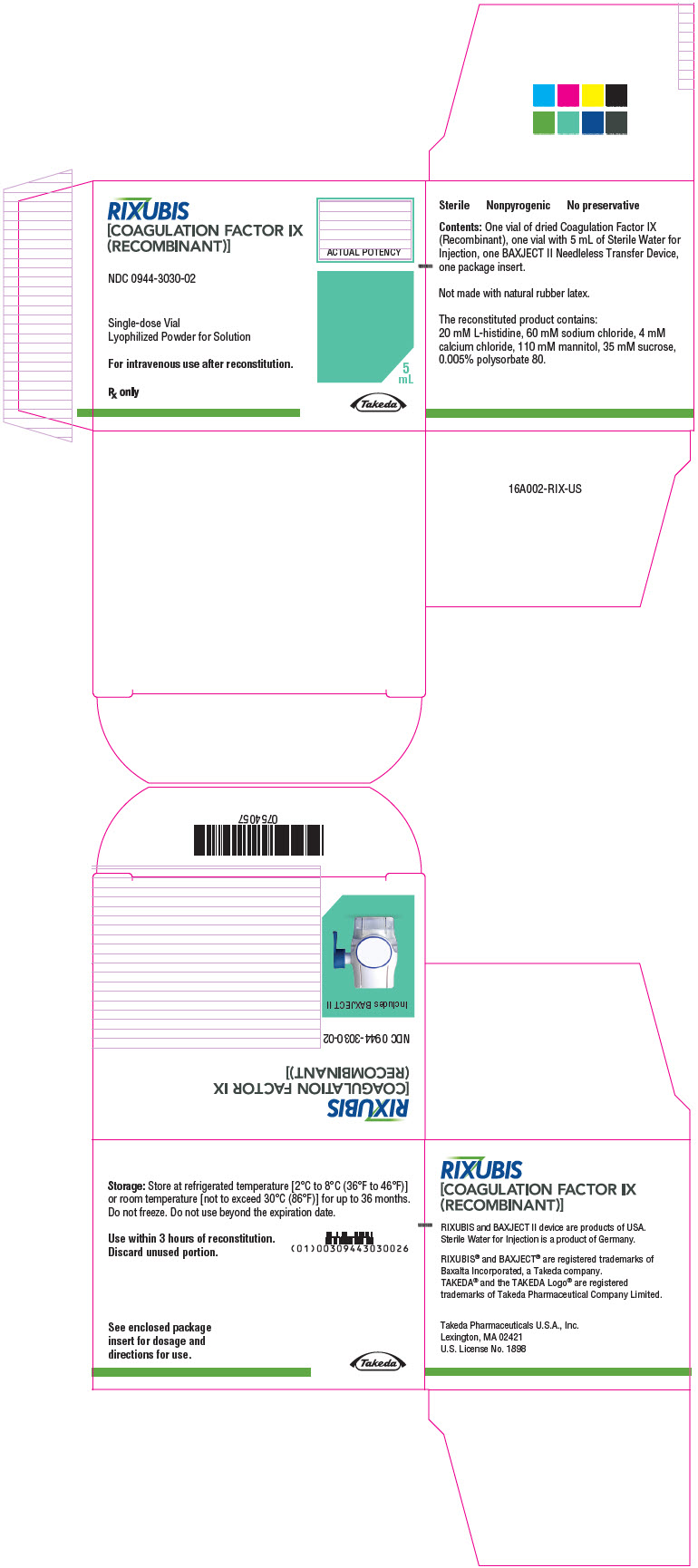
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 0944-3030-02
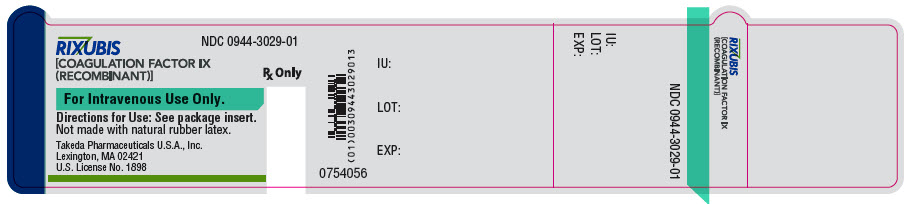
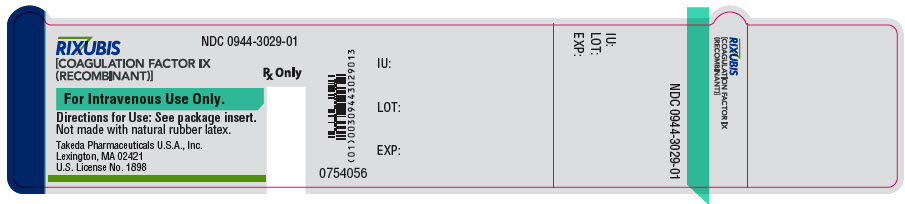
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 0944-3029-01
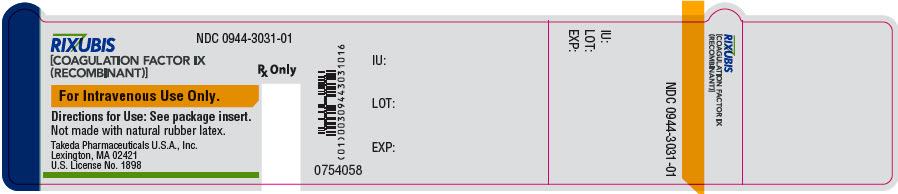
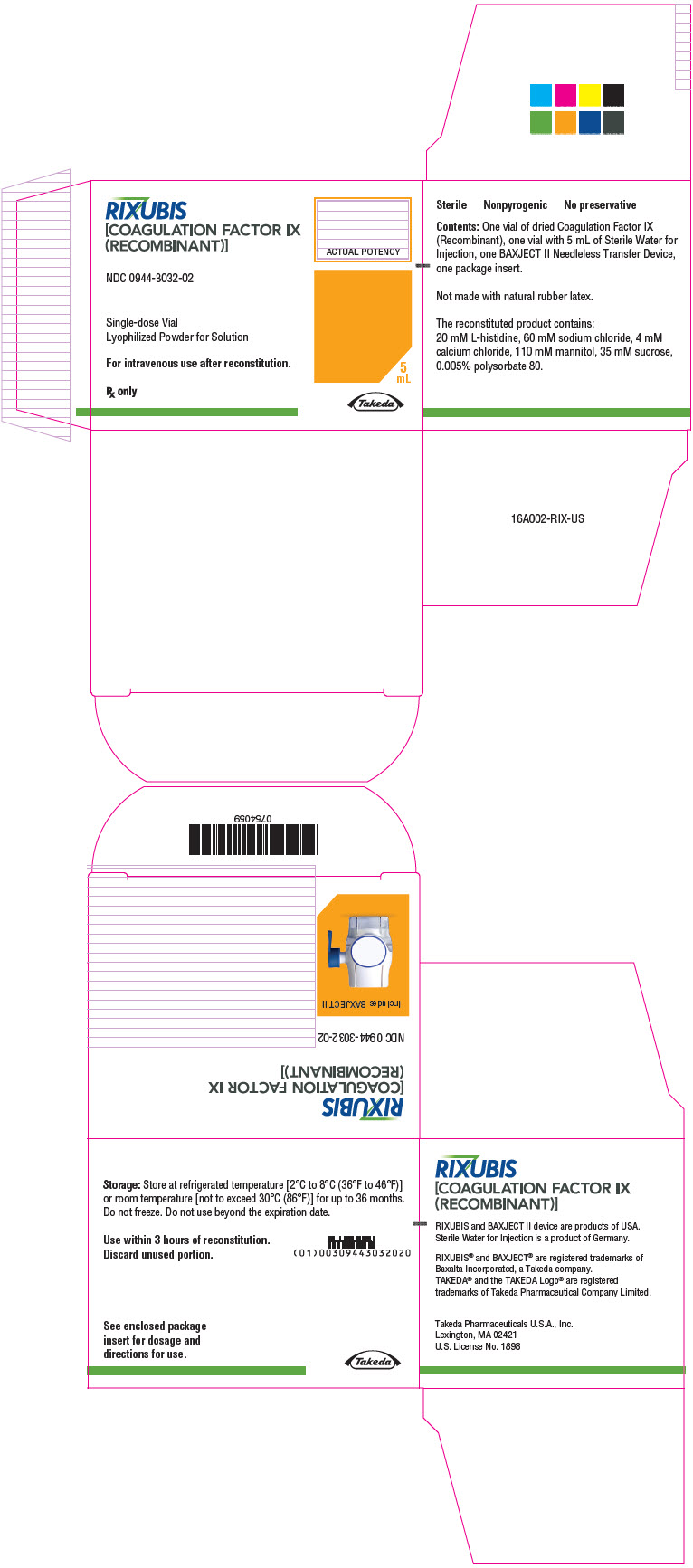
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 0944-3032-02
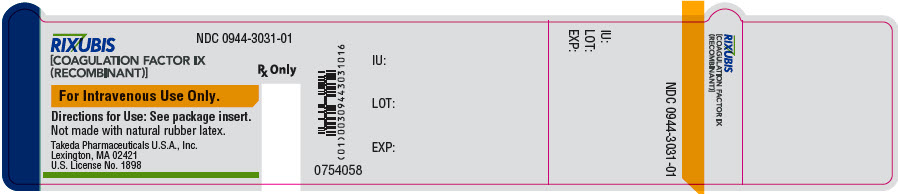
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 0944-3031-01
- PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 0944-3034-02
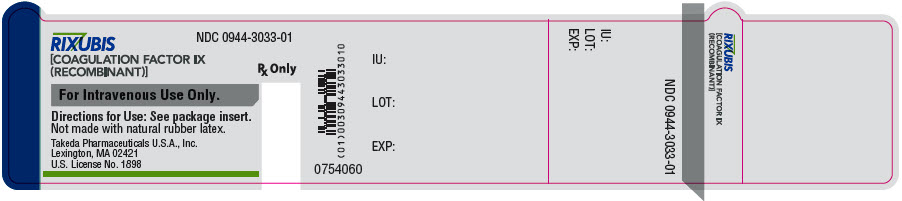
- PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 0944-3033-01
-
PRINCIPAL DISPLAY PANEL - 5 mL Vial Label - NDC 64764-515-50
5 mL
NDC 64764-515-50Takeda
Sterile Water for Injection, USP
for reconstitution of accompanying productDo not use unless clear. No antimicrobial agent or other substance
has been added. Do not use for intravascular injection without making
approximately isotonic by addition of suitable solute.
Discard unused portion.Rx Only
0754013
Single-dose container
Nonpyrogenic
-
INGREDIENTS AND APPEARANCE
RIXUBIS
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-3026 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3026-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 RIXUBIS
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-3025 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 250 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3025-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 10/04/2013 RIXUBIS
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-3028 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3028-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 RIXUBIS
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-3027 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 500 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3027-01 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 10/04/2013 RIXUBIS
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-3030 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3030-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 RIXUBIS
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-3029 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 1000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3029-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 10/04/2013 RIXUBIS
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-3032 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3032-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 RIXUBIS
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-3031 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 2000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3031-01 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 10/04/2013 RIXUBIS
coagulation factor ix (recombinant) kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:0944-3034 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3034-02 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 mL Part 2 1 VIAL, GLASS 5 mL Part 1 of 2 RIXUBIS
coagulation factor ix recombinant human injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC:0944-3033 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COAGULATION FACTOR IX RECOMBINANT HUMAN (UNII: 382L14738L) (COAGULATION FACTOR IX RECOMBINANT HUMAN - UNII:382L14738L) COAGULATION FACTOR IX RECOMBINANT HUMAN 3000 [iU] in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) SODIUM CHLORIDE (UNII: 451W47IQ8X) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MANNITOL (UNII: 3OWL53L36A) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0944-3033-01 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Part 2 of 2 STERILE WATER
water liquidProduct Information Item Code (Source) NDC:64764-515 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 5 mL in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-515-50 5 mL in 1 VIAL, GLASS; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 06/26/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125446 10/04/2013 Labeler - Takeda Pharmaceuticals America, Inc. (039997266) Establishment Name Address ID/FEI Business Operations BAXALTA US INC. 009471603 MANUFACTURE(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034) , LABEL(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034) , PACK(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034) Establishment Name Address ID/FEI Business Operations Siegfried Hameln GmbH 315869123 MANUFACTURE(64764-515) , ANALYSIS(64764-515) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Austria AG 300466733 ANALYSIS(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034) Establishment Name Address ID/FEI Business Operations Takeda Manufacturing Austria AG 300434670 ANALYSIS(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034) Establishment Name Address ID/FEI Business Operations OFI Technologie & Innovation GmbH 300681124 ANALYSIS(0944-3026, 0944-3028, 0944-3030, 0944-3032, 0944-3034)