Label: PIRSUE- pirlimycin hydrochloride injection, solution
- NDC Code(s): 54771-7688-1, 54771-7688-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated August 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Pirlimycin hydrochloride is a lincosaminide antibiotic.
Chemical Structure of Pirlimycin Hydrochloride 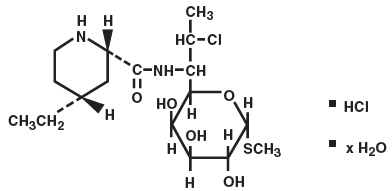
Chemical Name of Pirlimycin Hydrochloride Methyl(2S-Cis)-7-chloro-6,7,8-trideoxy-6[[(4-ethyl-2-piperidinyl)carbonyl]amino]-1-thio-L-threo-α-D-galacto-octo-pyranoside monohydrochloride hydrate.
PIRSUE Sterile Solution is a clear solution.
Each 10 mL PLASTET® Disposable Syringe contains: Pirlimycin free base equivalents (as the hydrochloride salt) 50 mg Anhydrous citric acid 42.3 mg Sodium citrate 88.2 mg Water for injection
q.s. -
INDICATIONS FOR USE
PIRSUE Sterile Solution (pirlimycin hydrochloride intramammary infusion) is indicated for the treatment of clinical and subclinical mastitis in lactating dairy cattle associated with Staphylococcus species such as Staphylococcus aureus and Streptococcus species such as Streptococcus agalactiae, Streptococcus dysgalactiae, and Streptococcus uberis.
- DOSAGE
-
ADMINISTRATION
Teat End Preparation
Wash teats thoroughly with water containing a suitable dairy antiseptic. Dry the teats thoroughly. Milk out the udder completely. Using the alcohol pad provided, wipe the teat end of the affected quarters, using a separate pad for each teat. Allow sufficient time (at least 5 to 10 seconds) for the alcohol to dry. Use of protective gloves by persons applying treatment is recommended as part of aseptic infusion technique.
Important Considerations for Extended Therapy
For extended duration of therapy, infuse only quarters known to be infected with label pathogens. Do not concurrently infuse uninfected low SCC quarters of the same cow. Prepare the teats using the above instructions, and then infuse PIRSUE Sterile Solution using aseptic infusion technique and partial insertion (see diagram below).
Infusion
The Plastet disposable syringe is designed to provide the choice of either insertion of the full cannula as has traditionally been practiced, or insertion of no more than 1/8 inch of the cannula, as reported by Eberhart, R.J., et. al., 1987. Current Concepts of Bovine Mastitis, 3rd Edition, National Mastitis Council, Arlington, VA.
a. Full insertion: Remove the white end cap by pulling straight up as shown. Gently insert the full cannula into the teat canal.
b. Partial insertion: Remove the white end cap by pulling straight up as shown. Gently insert the exposed white tip into the teat canal.
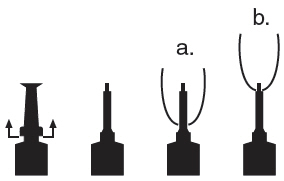
Choose the desired insertion length (full or partial) and gently insert the tip into the teat canal. Carefully push the plunger to infuse the entire contents, and then massage the quarter to distribute the solution into the milk cistern. Following infusion, dip all quarters with an antiseptic teat dip. Cows with systemic clinical signs caused by mastitis should receive other appropriate therapy under the direction of a licensed veterinarian.
.
-
WARNING
Repeated infusion during extended duration therapy regimens, even with adequate teat end preparation and sanitation, can result in elevated somatic cell counts and/or clinical mastitis, which can result in animal death. If acute clinical mastitis or other clinical signs of illness develop during extended duration therapy with PIRSUE, discontinue therapy immediately and contact your veterinarian.
Discard Empty Container; DO NOT REUSE
KEEP OUT OF REACH OF CHILDREN -
RESIDUE WARNINGS
- Milk taken from animals during treatment and for 36 hours after the last treatment must not be used for food regardless of treatment duration.
- Following infusion twice at a 24-hour interval, treated animals must not be slaughtered for 9 days.
- Following any extended duration of therapy (infusion longer than twice at a 24-hour interval, up to 8 consecutive days), animals must not be slaughtered for 21 days.
- Use of this product in a manner other than indicated under DOSAGE might result in violative residues.
- PRECAUTION
-
ADVERSE REACTIONS
As demonstrated in the pivotal target animal safety study, even with adequate pre-treatment preparation, repeated infusion of PIRSUE Sterile Solution resulted in elevated SCC and clinical mastitis due to infection with Gram-negative environmental pathogens. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae. For technical assistance, to report suspected adverse reactions, or to request a Safety Data Sheet (SDS), call 1-888-963-8471.
-
MICROBIOLOGY
Pirlimycin is a lincosaminide antibiotic that has activity against Gram-positive mastitis pathogens. Pirlimycin functions by binding to the 50S ribosomal subunit of bacterial ribonucleic acid, which interferes with protein synthesis within the bacteria. In vitro activity of pirlimycin has been demonstrated against Staphylococcus aureus, Streptococcus agalactiae, Streptococcus dysgalactiae, and Streptococcus uberis, four pathogens associated with clinical and subclinical mastitis in lactating dairy cattle.
Utilizing data that included isolates from cows with mastitis, zone diameter interpretive criteria and minimum inhibitory concentration (MIC) breakpoints were determined using standardized procedures from the Clinical and Laboratory Standards Institute (CLSI, formerly National Committee of Clinical Laboratory Standards) M31-A2. The CLSI-accepted interpretive criteria for pirlimycin against Gram-positive mastitis pathogens are shown in Table 1
Table 1. CLSI-Accepted Interpretive Criteria for Pirlimycin Against Bovine Mastitis Pathogens*
Pathogen
Disk Potency
Zone Diameter Interpretive Standards (mm)
MIC Breakpoint
(µg/mL)S
R
S
R
Staphylococcus aureus
Streptococcus agalactiae
Streptococcus dysgalactiae
2 µg
≥13
≤12
≤2.0
≥4.0
Streptococcus uberis
S - Susceptible
R - Resistant* These interpretive criteria are only intended for use when CLSI M31-A2 performance standards are used to determine antimicrobial susceptibility.
-
EFFECTIVENESS
The effectiveness of pirlimycin was demonstrated in a field dose response study in lactating dairy cattle with clinical mastitis. Three investigators enrolled 486 cows from 39 herds. Cows with abnormal milk (clots, flakes) and with or without udder clinical signs (swelling, redness, or soreness) were enrolled and treated, regardless of the mastitis pathogen isolated or the pre-treatment somatic cell count. Cows were treated in the affected quarter(s) with 50, 100, or 200 mg of pirlimycin twice at a 24-hour interval. A non-treated control group was included. In this study, an individual quarter was cured if it had normal milk, no udder clinical signs, and if the milk was negative for any mastitis pathogen at 10 days post-treatment. If no bacteria were isolated pre-treatment, a decrease in somatic cell count was required. A cow was cured if all enrolled quarters in that cow were cured. All three treatment levels had significantly greater cow cure rates than the non-treated control group. Based on this study, the dose of 50 mg of pirlimycin per quarter administered twice at a 24-hour interval was determined to be the effective dose for the treatment of clinical mastitis.
-
ANIMAL SAFETY
Two pivotal studies addressing the safety of pirlimycin administered at dosages of 50 mg or 200 mg (4X) into all four quarters twice at a 24-hour interval indicate that the formulation is safe and non-irritating to the bovine udder. Safety observations were also made during the clinical effectiveness study. No udder irritation was noted due to intramammary infusion with pirlimycin during these studies.
An additional study was conducted to determine the safety of extended duration therapy. Twenty lactating Holstein cows, first lactation or greater, at various milk production levels, and with no evidence of clinical mastitis were enrolled and treated with pirlimycin administered at a dosage of 50 mg/quarter in all four quarters daily for eight consecutive days. Cows were monitored for general health, changes in milk production and quality, and signs of udder irritation for a total of 14 days, beginning three days prior to the first treatment. Milk production was not affected by treatment. SCCs of treated cows were statistically significantly increased post-treatment relative to the pre-treatment level. A total of 24 pirlimycin-treated quarters (32%) in 15 cows had increased SCCs (>200,000 cells/mL) for at least two consecutive milkings. Of these, six treated cows (8 quarters) had a concurrent bacterial infection attributable to a mastitis pathogen. Udder irritation occurred in seven pirlimycin-treated cows (10 quarters). Abnormal strip cup scores occurred in six pirlimycin- treated cows (9 quarters). Most of the abnormal udder and strip cup observations were seen in quarters where bacteria were also isolated.
Corroborative data from field studies and field use reports indicate that although intramammary infusion of pirlimycin hydrochloride at 50 mg/quarter administered from two to eight consecutive days was well tolerated, repeated infusion with pirlimycin increases the potential for intramammary infections and subsequent clinical mastitis due to environmental bacteria, including coliform bacteria. Adverse reactions, including clinical signs of mastitis (udder swelling and abnormal milk), increased SCCs, and death from coliform mastitis have been reported in cows following extended therapy with pirlimycin. Some, but not all, adverse reactions were associated with failure to thoroughly clean quarters and to use aseptic infusion technique.
-
MILK AND TISSUE RESIDUE DEPLETION
The established tolerance of pirlimycin in milk is 0.40 ppm. Milk residue depletion studies were conducted in cows with clinical mastitis. In one study, cows were infused with 50 mg of pirlimycin twice at a 24-hour interval into all quarters regardless of the number of affected quarters. In a second study, cows with a single mastitic quarter were infused with 50 mg of pirlimycin twice at a 24-hour interval into only the affected quarter. In a third study, normal cows were infused with 50 mg of pirlimycin twice at a 24-hour interval into all four quarters. As a result of these three studies, milk taken from cows during treatment and for 36 hours following treatment must not be used for food and must be discarded. For extended duration of therapy (once daily for up to 8 consecutive days), a milk residue study was conducted where cows received 50 mg of pirlimycin per quarter into all four quarters for 8 consecutive days. This study confirmed that milk taken from cows during treatment and for 36 hours following the last treatment must not be used for food and must be discarded.
The established tolerance for pirlimycin in liver (the target tissue) is 0.5 ppm. A pivotal tissue residue study was conducted following administration of 50 mg of pirlimycin twice at a 24-hour interval into all four quarters. Following receipt of the 50 mg of pirlimycin twice at a 24-hour interval into all four quarters, the liver residue decline data from this study supports a 9-day pre-slaughter withdrawal period.
For extended duration of therapy, a second tissue residue study was conducted. Each lactating cow received 50 mg pirlimycin per quarter into all four quarters, once daily for 8 consecutive days. Using the established tolerance for pirlimycin of 0.5 ppm in the liver, these data support a 21-day pre-slaughter withdrawal period for extended duration pirlimycin therapy. Extended duration of therapy is considered as any treatment period longer than 2 days (up to 8 consecutive days) of therapy.
-
EFFECT ON MILK MANUFACTURING STARTER CULTURES
A study was conducted to examine the effect of varying concentrations of pirlimycin in milk on the growth of bacterial starter cultures used to produce fermented milk products. Pirlimycin did not adversely affect bacterial starter cultures used for the production of fermented milk products at concentrations found following normal label use including proper milk discard periods. Violative levels of pirlimycin (>0.40 ppm) can adversely affect the growth of bacterial starter cultures.
- STORAGE CONDITIONS
-
HOW SUPPLIED
PIRSUE Sterile Solution is available in unbroken packages of 12-10 mL Plastet Disposable Syringes with 12 individually wrapped 70% isopropyl alcohol pads. The Plastet Disposable Syringes are packaged in Cartons (12-10 mL Plastet Disposable Syringes per carton) and in Pails (12 packages of 12-10 mL Plastet Disposable Syringes or 144 Plastets per pail).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Pirsue Carton Label
- PRINCIPAL DISPLAY PANEL - Pirsue Container Label
-
INGREDIENTS AND APPEARANCE
PIRSUE
pirlimycin hydrochloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-7688 Route of Administration INTRAMAMMARY Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIRLIMYCIN HYDROCHLORIDE (UNII: 8S09O559AQ) (PIRLIMYCIN - UNII:LM19JT6G5K) PIRLIMYCIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 4.23 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) 8.82 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-7688-1 12 in 1 CARTON 1 10 mL in 1 SYRINGE 2 NDC:54771-7688-2 144 in 1 PAIL 2 10 mL in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141036 09/07/2000 Labeler - Zoetis Inc. (828851555)




