Label: CESTEX- epsiprantel tablet, film coated
-
NDC Code(s):
54771-8032-1,
54771-8032-2,
54771-8033-1,
54771-8033-2, view more54771-8034-1, 54771-8034-2, 54771-8035-1, 54771-8035-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 2, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Veterinary Tablets
- CAUTION
-
DESCRIPTION
Cestex tablets are film-coated and contain 12.5 mg, 25 mg, 50 mg or 100 mg of epsiprantel per tablet.
Epsiprantel is an anthelmintic that is active as a single dose against the common tapeworms of cats and dogs. Epsiprantel has a molecular weight of 326 and is chemically 2-(cyclohexyl-carbonyl)-4-oxo 1,2,3,4,6,7,8,12b-octahydropyrazino[2,1-a][2]benza-zepine. It is a stable white solid which is sparingly soluble in water. Its chemical structure is presented below.
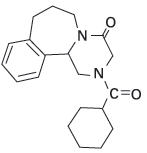
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTION
-
DOSAGE AND ADMINISTRATION
Cestex tablets should be administered orally.
The recommended dosage of epsiprantel is: cats, 1.25 mg/lb of body weight; dogs, 2.5 mg/lb of body weight. The following table may be used as a guide:
Dosage Schedule Feline Body Weight Dose Seven weeks old up to 10 lb 12.5 mg 11–20 lb 25.0 mg Canine Body Weight Dose Seven weeks old up to 5 lb 12.5 mg 6–10 lb 25.0 mg 11–20 lb 50.0 mg 21–40 lb 100.0 mg 41–50 lb 125.0 mg 51–60 lb 150.0 mg 61–80 lb 200.0 mg 81–90 lb 225.0 mg 91–100 lb 250.0 mg 101+ lb 2.5 mg/lb, rounding up to next whole tablet combination Fasting is not necessary or recommended.
Unless exposure to the infected intermediate hosts is controlled, reinfection is likely and retreatment may be required. In the case of D. caninum, an effective flea control program should be instituted.
-
SAFETY
Epsiprantel has been evaluated in cats at 5 times the recommended dose given once daily for 3 days with no adverse effects noted. In tolerance studies, epsiprantel produced minimal clinical signs in cats given 40 times the recommended dose once daily for 4 days.
Epsiprantel has been evaluated in 14-day repeat dose studies in dogs at 500 mg/kg (90 times recommended dosage) with no significant adverse results. No side effects were observed during the clinical field studies.
Epsiprantel is not a cholinesterase inhibitor. During the course of clinical field studies, Cestex was administered concurrently with diethylcarbamazine citrate (dogs only), anti-inflammatory agents, insecticides, and nematocides with no drug incompatibilities noted.
- STORAGE
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 12.5 mg Bottle Label
- PRINCIPAL DISPLAY PANEL - 25 mg Bottle Label
- PRINCIPAL DISPLAY PANEL - 50 mg Bottle Label
- PRINCIPAL DISPLAY PANEL - 100 mg Bottle Label
-
INGREDIENTS AND APPEARANCE
CESTEX
epsiprantel tablet, film coatedProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epsiprantel (UNII: 0C1SPQ0FSR) (epsiprantel - UNII:0C1SPQ0FSR) epsiprantel 12.5 mg Product Characteristics Color RED Score 2 pieces Shape ROUND (bi-convex) Size 6mm Flavor Imprint Code 219 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8032-1 50 in 1 BOTTLE 2 NDC:54771-8032-2 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140893 12/01/1989 CESTEX
epsiprantel tablet, film coatedProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epsiprantel (UNII: 0C1SPQ0FSR) (epsiprantel - UNII:0C1SPQ0FSR) epsiprantel 25 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND (bi-convex) Size 8mm Flavor Imprint Code 220 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8033-1 50 in 1 BOTTLE 2 NDC:54771-8033-2 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140893 12/01/1989 CESTEX
epsiprantel tablet, film coatedProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epsiprantel (UNII: 0C1SPQ0FSR) (epsiprantel - UNII:0C1SPQ0FSR) epsiprantel 50 mg Product Characteristics Color GRAY Score 2 pieces Shape ROUND (bi-convex) Size 10mm Flavor Imprint Code 221 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8034-1 25 in 1 BOTTLE 2 NDC:54771-8034-2 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140893 12/01/1989 CESTEX
epsiprantel tablet, film coatedProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8035 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epsiprantel (UNII: 0C1SPQ0FSR) (epsiprantel - UNII:0C1SPQ0FSR) epsiprantel 100 mg Product Characteristics Color ORANGE (peach) Score 2 pieces Shape ROUND (bi-convex) Size 13mm Flavor Imprint Code 222 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8035-1 25 in 1 BOTTLE 2 NDC:54771-8035-2 50 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140893 12/01/1989 Labeler - Zoetis Inc. (828851555)








