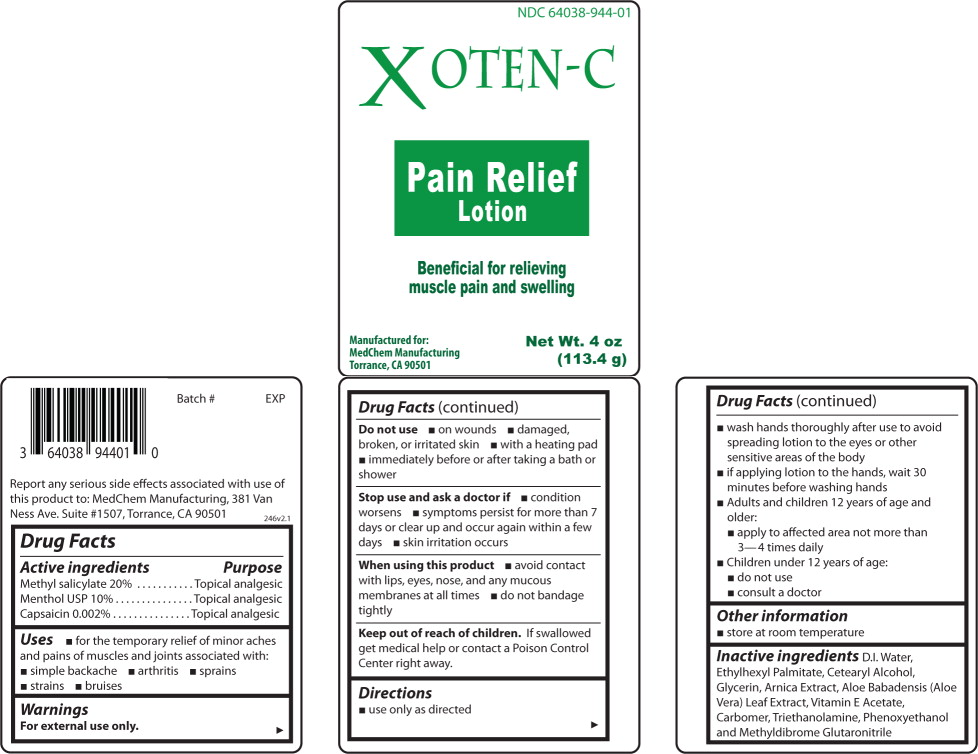
Label: XOTEN-C- methyl salicylate, menthol, capsaicin lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 64038-944-01 - Packager: Living Well Pharmacy Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Use
-
Warnings
For external use only.
Do not use
- on wounds
- damaged, broken, or irritated skin
- with a heating pad
- immediately before or after taking a bath or shower
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- skin irritation occurs
-
Directions
- use only as directed
- Wash hands thoroughly after use to avoid spreading lotion to the eyes or other sensitive areas of the body
- if applying lotion to the hands, wait 30 minutes before washing hands
- Adults and children 12 years of age and older:
- apply to affected area not more than 3-4 times daily
- Children under 12 years of age:
- do not use
- consult a doctor
- Other information
- Inactive ingredients
- Principal Display Panel – Bottle Label
-
INGREDIENTS AND APPEARANCE
XOTEN-C
methyl salicylate, menthol, capsaicin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64038-944 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methyl salicylate (UNII: LAV5U5022Y) (Methyl salicylate - UNII:LAV5U5022Y) Methyl salicylate 20 g in 100 g Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol 10 g in 100 g Capsaicin (UNII: S07O44R1ZM) (Capsaicin - UNII:S07O44R1ZM) Capsaicin 0.002 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Ethylhexyl Palmitate (UNII: 2865993309) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Glycerin (UNII: PDC6A3C0OX) ARNICA MONTANA (UNII: O80TY208ZW) Aloe Vera Leaf (UNII: ZY81Z83H0X) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) Phenoxyethanol (UNII: HIE492ZZ3T) METHYLDIBROMO GLUTARONITRILE (UNII: YX089CPS05) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64038-944-01 113.4 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/01/2011 Labeler - Living Well Pharmacy Inc. (070488957)