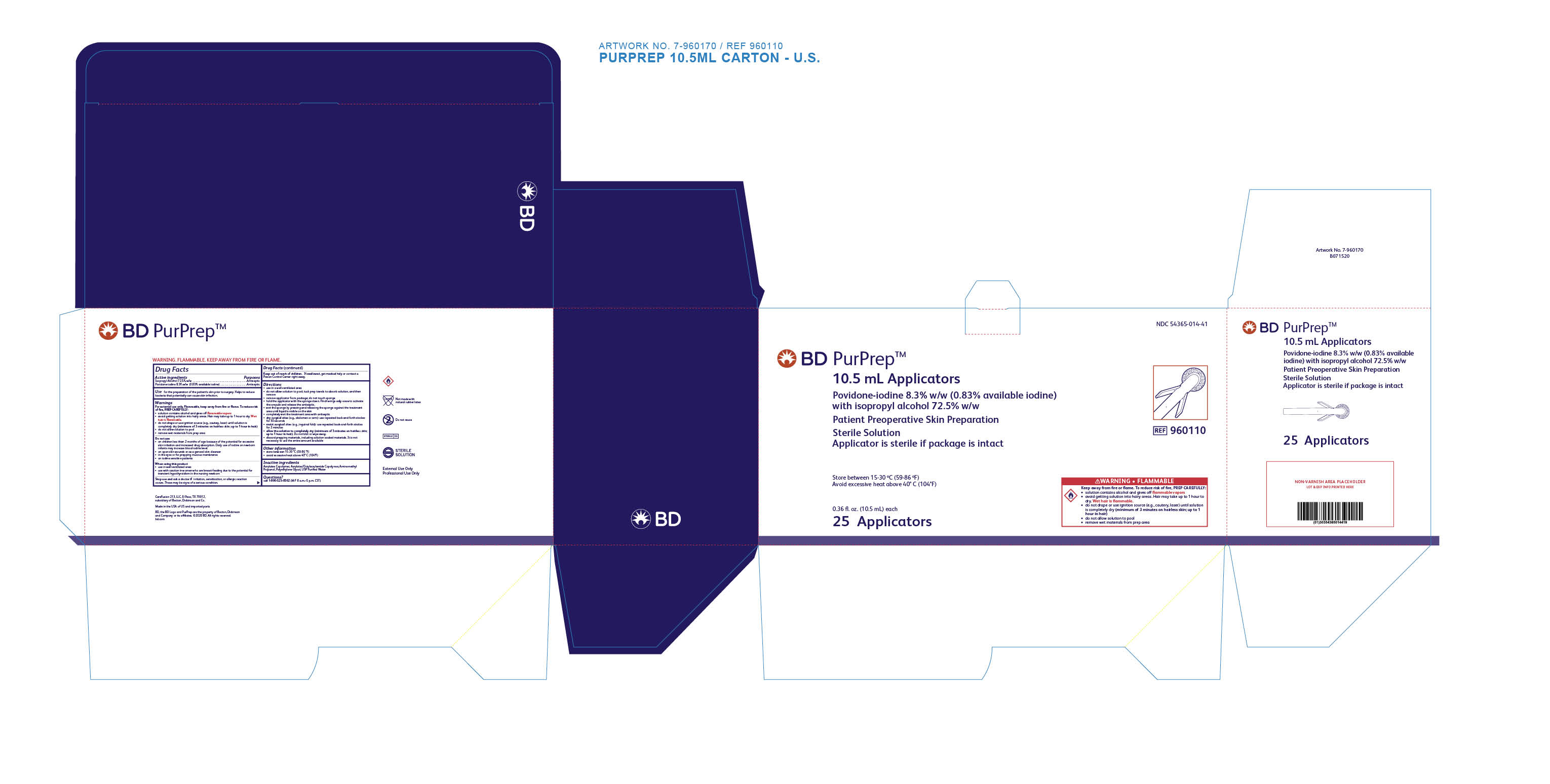
Label: PURPREP- povidone iodine and isopropyl alcohol sponge
- NDC Code(s): 54365-014-41, 54365-014-42
- Packager: CareFusion 213 LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only. Flammable, keep away from fire or flame.
T o reduce risk of fire, PREP CAREFULLY:
- do not use 26-mL applicator for head and neck surgery
- solution contains alcohol and gives off flammable vapors.
- avoid getting solution into hairy areas. Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery, laser) until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair)
- do not allow the solution to pool
- remove wet materials from prep area
-
DO NOT USE
Do not use
- on children less than 2 months of age because of the potential for excessive skin irritation and increased drug absorption. Daily use of iodine on newborn infants may increase blood iodine level.
- on open skin wounds or as a general skin cleanser
- in the eyes or for prepping mucous membranes
- on iodine sensitive patients
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
-
- use in a well ventilated area
- do not allow solution to pool; tuck prep towels to absorb solution, and then remove
- remove applicator from package; do not touch sponge
- hold the applicator with the sponge down. Pinch wings only once to activate the ampules and release the antiseptic.
- wet the sponge by pressing and releasing the sponge against the treatment area until liquid is visible on the skin
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): use repeated back-and-forth strokes for 30 seconds
- moist surgical sites (e.g., inguinal fold): use repeated back-and-forth strokes for 2 minutes
- allow the solution to completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair). Do not blot or wipe away.
- discard prepping materials, including solution soaked materials. It is not necessary to use the entire amount available
-
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURPREP
povidone iodine and isopropyl alcohol spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54365-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 725 mg in 1 mL POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 8.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54365-014-42 1 in 1 POUCH 04/09/2020 1 26 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:54365-014-41 1 in 1 POUCH 05/21/2020 2 10.5 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/09/2020 Labeler - CareFusion 213 LLC (826496312) Registrant - Becton, Dickinson and Company (832696038) Establishment Name Address ID/FEI Business Operations Becton, Dickinson and Company 124987988 manufacture(54365-014) Establishment Name Address ID/FEI Business Operations CareFusion 213 LLC 826496312 manufacture(54365-014) , pack(54365-014) , label(54365-014) , sterilize(54365-014) , analysis(54365-014)