Label: GENTAMICIN PIGLET- gentamicin sulfate injection
- NDC Code(s): 58005-663-04, 58005-663-05, 58005-663-06
- Packager: Sparhawk Laboratories, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated June 10, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
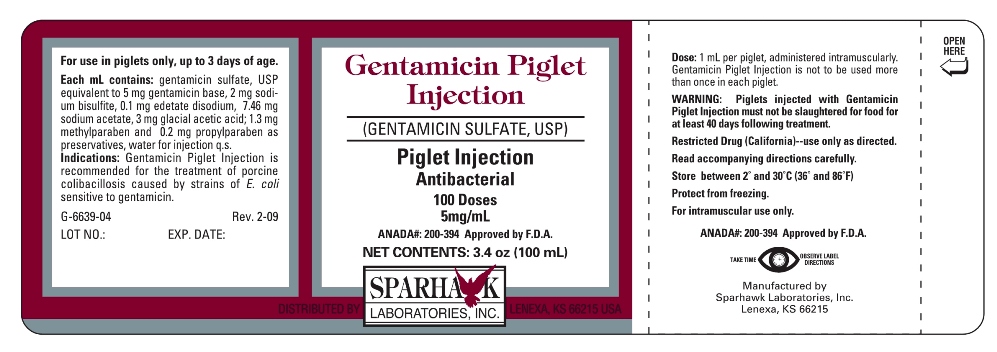
DESCRIPTION
Gentamicin Piglet Injection is a sterile preparation for intramuscular administration in piglets up to 3 days of age. Each milliliter contains gentamicin sulfate, USP equivalent to 5 mg gentamicin base, 2 mg sodium bisulfite, 0.1 mg edetate disodium, 7.46 mg sodium acetate, 3 mg glacial acetic acid; 1.3 mg methylparaben and 0.2 mg propylparaben as preservatives, water for injection q.s.
-
WARNING
For use in piglets up to 3 days of age only.
Piglets injected with Gentamicin Piglet Injection must not be slaughtered for food for at least forty (40) days following treatment. Use of more than the recommended dose will result in gentamicin tissue residues beyond forty (40) days.
Restricted Drug (California)--use only as directed.
Read accompanying directions carefully.
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
The recommended dose is 1 mL (5 mg) administered intramuscularly. Gentamicin Piglet Injection is not to be used more than once in each piglet. If piglets do not respond to treatment with Gentamicin Piglet Injection, diagnosis should be reevaluated. Clean and sterilize needles and syringes by boiling in water for 15 minutes prior to use. Prior to injection, disinfect the injection site and top the vial with a suitable disinfectant, such as 70% alcohol. Use all precautions to prevent contamination of vial contents.
- CONTRAINDICATIONS
- PRECAUTIONS
-
CHEMISTRY
Gentamicin is a mixture of aminoglycoside antibiotics derived from the fermentation of Micromonospora purpurea. Gentamicin Sulfate, USP is a mixture of sulfate salts of the antibiotics produced in the fermentation. The salts are weakly acidic and freely soluble in water. Gentamicin sulfate, USP is stable in solution for the period of time indicated on the labels by the expiration date.
Gentamicin sulfate, USP contains not less than 500 micrograms of gentamicin base per milligram.
- HOW SUPPLIED
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GENTAMICIN PIGLET
gentamicin sulfate injectionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:58005-663 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GENTAMICIN SULFATE (UNII: 8X7386QRLV) (GENTAMICIN - UNII:T6Z9V48IKG) GENTAMICIN 5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58005-663-04 100 mL in 1 VIAL 2 NDC:58005-663-05 250 mL in 1 VIAL 3 NDC:58005-663-06 500 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200394 07/31/2007 Labeler - Sparhawk Laboratories, Inc. (147979082)