Label: OXYGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 66988-100-37 - Packager: Jenco Medical and Mobility, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 16, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
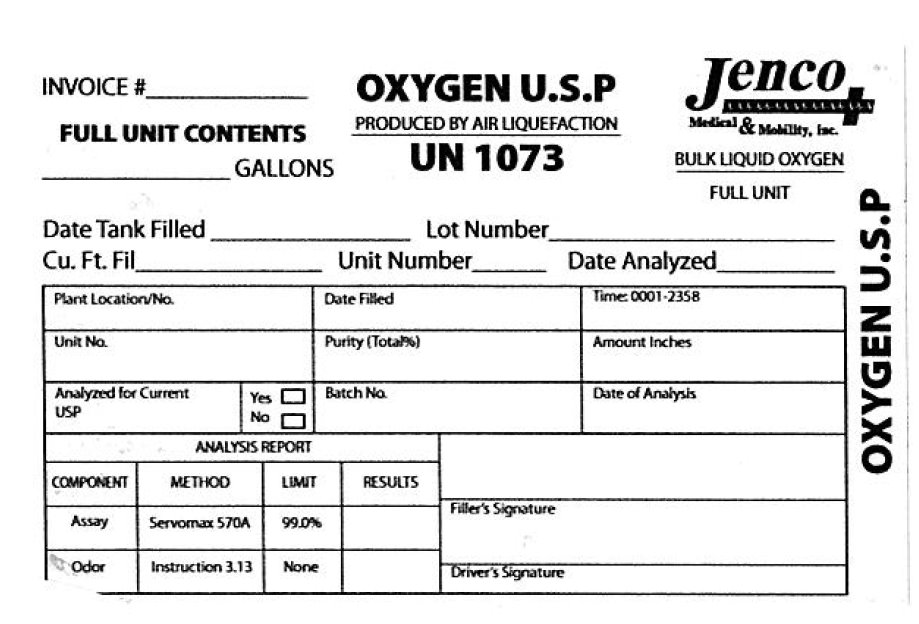
PRINCIPAL DISPLAY PANEL
Invoice OXYGEN U.S.P Jenco Medical and Mobility
Full Unit Contents Produced By Air Liquefaction Bulk Liquid Oxygen
Gallons UN 1073 Full Unit
Date Tank Filled Lot Number
Cu. Ft. Fil Unit Number Date Analyzed
Plant Location /No. Date Filled Time:0001-2358
Unit No. Purity Amount Inches
Analyzed for Current USP Batch No Date of Analysis
Analysis Report
Component Method Limit Results
Assay Servomax 570A 99.0 Percent
Odor Instruction 3.13 none
Filler's Signature
Driver's Signature - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66988-100 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 1 L in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66988-100-37 37 L in 1 CYLINDER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 06/14/2010 Labeler - Jenco Medical and Mobility, Inc (101297237) Registrant - Jenco Medical and Mobility, Inc (101297237) Establishment Name Address ID/FEI Business Operations Jenco Medical and Mobility, Inc 101297237 manufacture