Label: DICLOFENAC SODIUM solution
- NDC Code(s): 61314-014-05, 61314-014-25
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
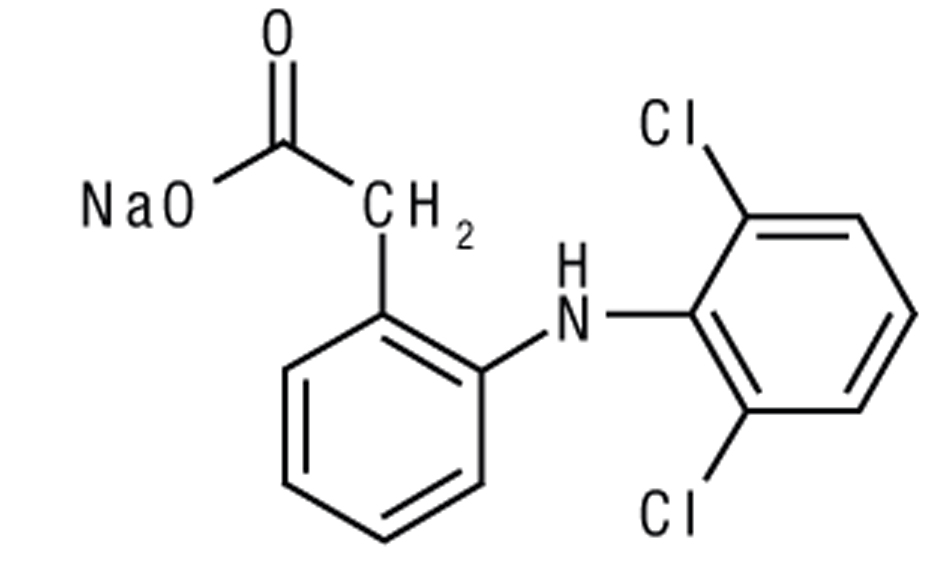
Diclofenac sodium ophthalmic solution, 0.1% is a sterile, topical, nonsteroidal, anti-inflammatory product for ophthalmic use. Diclofenac sodium is designated chemically as 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt, with an empirical formula of C14H10Cl2NO2Na. The structural formula of diclofenac sodium is:
Diclofenac sodium ophthalmic solution, 0.1% is available as a sterile solution which contains diclofenac sodium 0.1% (1 mg/mL).
Inactive Ingredients: polyoxyl 35 castor oil, boric acid, tromethamine, sorbic acid (2 mg/mL), edetate disodium (1 mg/mL), and purified water.
Diclofenac sodium is a faintly yellow-white to light-beige, slightly hygroscopic crystalline powder. It is freely soluble in methanol, sparingly soluble in water, very slightly soluble in acetonitrile, and insoluble in chloroform and in 0.1N hydrochloric acid. Its molecular weight is 318.14. Diclofenac sodium ophthalmic solution, 0.1% is an iso-osmotic solution with an osmolality of about 300 mOsmol/1000 g, buffered at approximately pH 7.2. Diclofenac sodium ophthalmic solution, 0.1% has a faint characteristic odor of castor oil.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
Diclofenac sodium is one of a series of phenylacetic acids that has demonstrated anti-inflammatory and analgesic properties in pharmacological studies. It is thought to inhibit the enzyme cyclooxygenase, which is essential in the biosynthesis of prostaglandins.
Animal Studies
Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed in animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
Pharmacokinetics
Results from a bioavailability study established that plasma levels of diclofenac following ocular instillation of two drops of diclofenac sodium ophthalmic solution, 0.1% to each eye were below the limit of quantification (10 ng/mL) over a 4-hour period. This study suggests that limited, if any, systemic absorption occurs with diclofenac sodium ophthalmic solution, 0.1%.
Clinical Trials
Postoperative Anti-Inflammatory Effects
In two double-masked, controlled, efficacy studies of postoperative inflammation, a total of 206 cataract patients were treated with diclofenac sodium ophthalmic solution, 0.1% and 103 patients were treated with vehicle placebo. Diclofenac sodium ophthalmic solution, 0.1% was favored over vehicle placebo over a 2-week period for the clinical assessments of inflammation as measured by anterior chamber cells and flare.
In double-masked, controlled studies of corneal refractive surgery (radial keratotomy (RK) and laser photorefractive keratectomy (PRK)) patients were treated with diclofenac sodium ophthalmic solution, 0.1% and/or vehicle placebo. The efficacy of diclofenac sodium ophthalmic solution given before and shortly after surgery was favored over vehicle placebo during the 6-hour period following surgery for the clinical assessments of pain and photophobia. Patients were permitted to use a hydrogel soft contact lens with diclofenac sodium ophthalmic solution for up to three days after PRK.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
The refractive stability of patients undergoing corneal refractive procedures and treated with diclofenac sodium ophthalmic solution has not been established. Patients should be monitored for a year following use in this setting. With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied non-steroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other nonsteroidal anti-inflammatory agents. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
-
PRECAUTIONS
General Precautions
All topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
Use of topical NSAIDs may result in keratitis. In some susceptible patients continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal infiltrates, corneal erosion, corneal ulceration, and corneal perforation. These events may be sight-threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients experiencing complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface disease (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events, which may become sight-threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days post-surgery may increase patient risk for occurrence and severity of corneal adverse events.
It is recommended that diclofenac sodium ophthalmic solution, like other NSAIDs, be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time. Use of the same bottle for both eyes is not recommended with topical eye drops that are used in association with surgery.
Results from clinical studies indicate that diclofenac sodium ophthalmic solution has no significant effect upon ocular pressure. However, elevations in intraocular pressure may occur following cataract surgery.
Information for Patients
Except for the use of a bandage hydrogel soft contact lens during the first 3 days following refractive surgery, diclofenac sodium ophthalmic solution should not be used by patients currently wearing soft contact lenses due to adverse events that have occurred in other circumstances.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in rats given diclofenac sodium ophthalmic solution in oral doses up to 2 mg/kg/day (approximately 500 times the human topical ophthalmic dose) revealed no significant increases in tumor incidence. A 2-year carcinogenicity study conducted in mice employing oral diclofenac sodium ophthalmic solution up to 2 mg/kg/day did not reveal any oncogenic potential. Diclofenac sodium ophthalmic solution did not show mutagenic potential in various mutagenicity studies including the Ames test. Diclofenac sodium ophthalmic solution administered to male and female rats at 4 mg/kg/day (approximately 1000 times the human topical ophthalmic dose) did not affect fertility.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger adult patients.
Teratogenic Effects
Pregnancy Category C.Reproduction studies performed in mice at oral doses up to 5,000 times (20 mg/kg/day) and in rats and rabbits at oral doses up to 2,500 times (10 mg/kg/day) the human topical dose have revealed no evidence of teratogenicity due to diclofenac sodium ophthalmic solution despite the induction of maternal toxicity and fetal toxicity. In rats, maternally toxic doses were associated with dystocia, prolonged gestation, reduced fetal weights and growth, and reduced fetal survival. Diclofenac sodium ophthalmic solution has been shown to cross the placental barrier in mice and rats.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Non-teratogenic Effects
Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetal cardiovascular system (closure of ductus arteriosus), the use of diclofenac sodium ophthalmic solution during late pregnancy should be avoided.
Nursing Women
It is not known whether topical ophthalmic administration of diclofenac sodium ophthalmic solution, 0.1% could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Ocular
Transient burning and stinging were reported in approximately 15% of patients across studies with the use of diclofenac sodium ophthalmic solution. In cataract surgery studies, keratitis was reported in up to 28% of patients receiving diclofenac sodium ophthalmic solution, although in many of these cases keratitis was initially noted prior to the initiation of treatment. Elevated intraocular pressure following cataract surgery was reported in approximately 15% of patients undergoing cataract surgery.
Lacrimation complaints were reported in approximately 30% of case studies undergoing incisional refractive surgery. The following adverse reactions were reported in approximately 10% or less of patients: abnormal vision, acute elevated IOP, blurred vision, conjunctivitis, corneal deposits, corneal edema, corneal opacity, corneal lesions, discharge, eyelid swelling, eye pain, injection (redness), iritis, irritation, itching, lacrimation disorder, and ocular allergy.
Systemic
The following adverse reactions were reported in 3% or less of patients: abdominal pain, asthenia, chills, dizziness, facial edema, fever, headache, insomnia, nausea, pain, rhinitis, viral infection, and vomiting.
Clinical Practice
The following reactions have been identified during postmarketing use of topical diclofenac sodium ophthalmic solution, 0.1% in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to topical diclofenac sodium ophthalmic solution, 0.1%, or a combination of these factors, include corneal erosion, corneal infiltrates, corneal perforation, corneal thinning, corneal ulceration, and epithelial breakdown [see PRECAUTIONS, General].
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Cataract Surgery
One drop of diclofenac sodium ophthalmic solution should be applied to the affected eye, 4 times daily beginning 24 hours after cataract surgery and continuing throughout the first 2 weeks of the postoperative period.
Corneal Refractive Surgery
One or two drops of diclofenac sodium ophthalmic solution should be applied to the operative eye within the hour prior to corneal refractive surgery. Within 15 minutes after surgery, one or two drops should be applied to the operative eye and continued 4 times daily for up to 3 days.
-
HOW SUPPLIED
Diclofenac sodium ophthalmic solution, 0.1% (1 mg/mL) Sterile Solution is supplied in a low density polyethylene (LDPE) white bottle with a natural LDPE dropper tip and a gray polypropylene closure in the following sizes:
2.5 mL fill in 4 mL container NDC 61314-014-25
5 mL fill in 8 mL container NDC 61314-014-05
STORAGE CONDITIONS
Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F).
Protect from light.
Dispense in original, unopened container only.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DICLOFENAC SODIUM
diclofenac sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61314-014 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) BORIC ACID (UNII: R57ZHV85D4) TROMETHAMINE (UNII: 023C2WHX2V) SORBIC ACID (UNII: X045WJ989B) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61314-014-25 2.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2008 2 NDC:61314-014-05 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078031 02/14/2008 Labeler - Sandoz Inc (005387188)