Label: OXYGEN gas
-
Contains inactivated NDC Code(s)
NDC Code(s): 52200-001-01, 52200-001-02, 52200-001-03, 52200-001-04, view more52200-001-05, 52200-001-06 - Packager: Home Care Medical LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 1, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
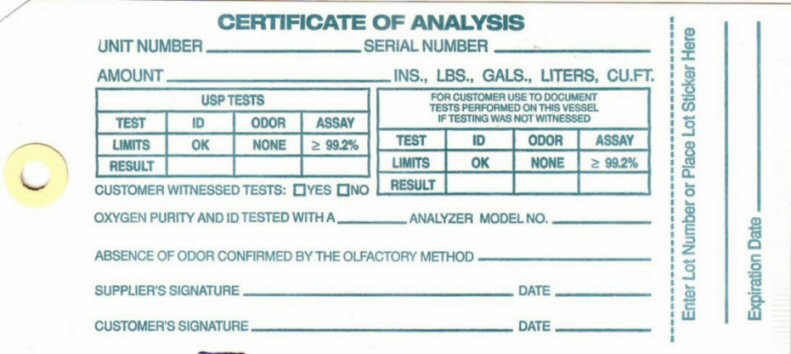
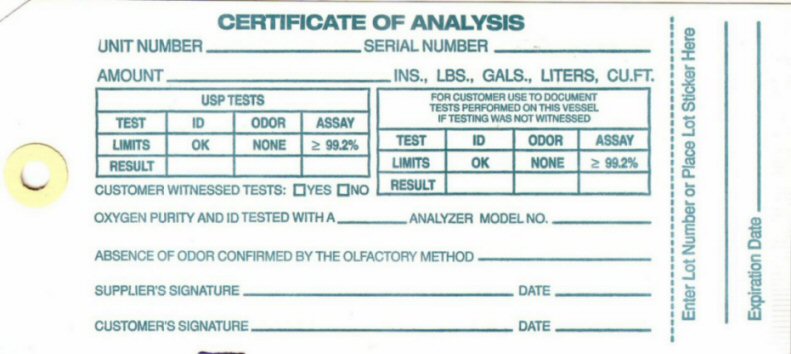
Certificate of Analysis
CERTIFICATE OF ANALYSIS UNIT NUMBER___________ SERIAL NUMBER___________ AMOUNT_________________INS, LBS, LITERS, CU FT.
USP TESTS
TEST ID ODOR ASSAY
LIMITS OK NONE 99.2%
RESULT __ _____ _____
FOR CUSTOMERS USE TO DOCUMENT TESTS PERFORMED ON THIS VESSEL IF TESTING WAS NOT WITNESSED
TEST ID ODOR ASSAY
LIMITS OK NONE 99.2%
RESULT __ _____ _____
CUSTOMER WITNESSED TESTS? _YES ___NO
OXYGEN PURITY AND ID TESTED WITH A __________ ANALYZER MODEL NO_________ ABSENCE OF ODOR CONFIRMED BY THE OLIFACTORY METHOD _________ SUPPLIERS SIGNATURE_____________________ DATE____________ CUSTOMERS SIGNATURE_____________DATE____________ ENTER LOT NUMBER AND PLACE STICKER HERE________________ EXPIRATION DATE__________
MEDICAL GASES LIQUID OXYGEN U.S.P. DELIVERY TAG FILLED BY: (PLEASE STAMP LOCATION ADDRESS OR PLACE LABEL BELOW) THIS VESSEL CONTAINS OXYGEN U.S.P. (SEE REVERSE SIDE FOR TEST RESULTS) OXYGEN PRODUCED BY THE AIR LIQUEFACTION METHOD

-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52200-001 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52200-001-01 21 L in 1 DEWAR 2 NDC:52200-001-02 31 L in 1 DEWAR 3 NDC:52200-001-03 36 L in 1 DEWAR 4 NDC:52200-001-04 41 L in 1 DEWAR 5 NDC:52200-001-05 45 L in 1 DEWAR 6 NDC:52200-001-06 46 L in 1 DEWAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved medical gas 01/01/1981 Labeler - Home Care Medical LLC (043751499) Registrant - Home Care Medical LLC (043751499) Establishment Name Address ID/FEI Business Operations Home Care Medical LLC 043751499 manufacture