Label: OXYGEN gas
- NDC Code(s): 42914-001-01
- Packager: Aspen Air U.S., LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
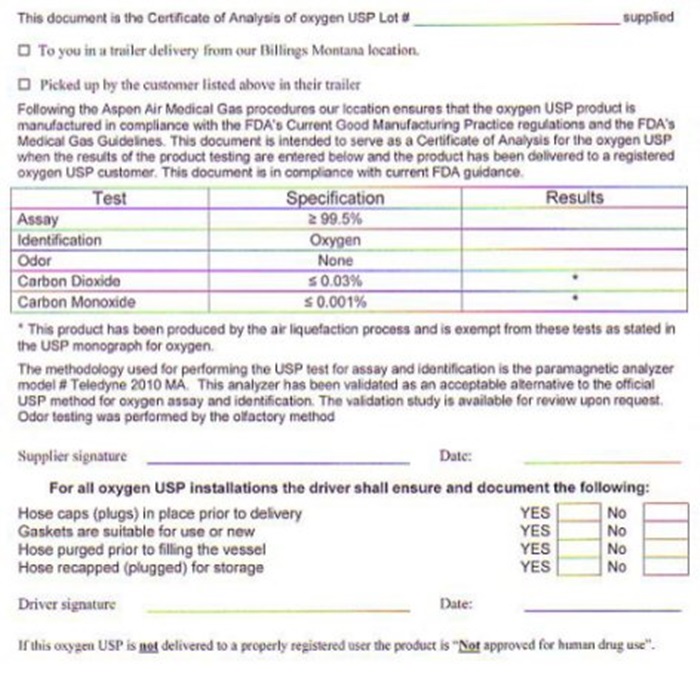
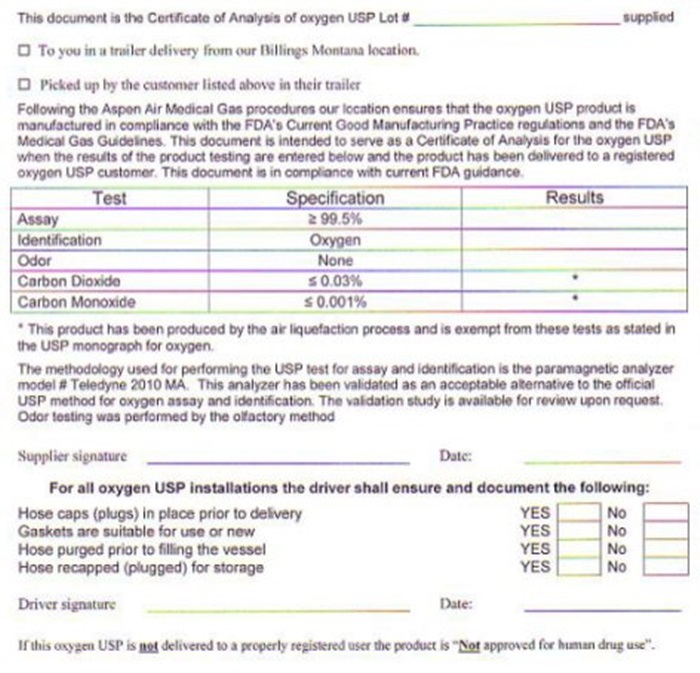
OXYGEN CERTIFICATE OF ANALYSIS
THIS DOCUMENT IS THE CERTIFICATE OF ANALYSIS OF OXYGEN USP LOT # ______________________ SUPPLIED TO YOU IN A TRAILER FROM OUR BILLINGS MONTANA LOCATION OR PICKED UP BY THE CUSTOMER LISTED ABOVE IN THEIR TRAILER. FOLLOWING THE ASPEN AIR MEDICAL GAS PROCEDURES OUR LOCATION ENSURES THAT THE OXYGEN USP PRODUCT IS MANUFACTURED IN COMPLIANCE WITH THE FDA'S CURRENT GOOD MANUFACTURING PRACTICE REGULATIONS AND THE FDA'S MEDICAL GAS GUIDELINES. THIS DOCUMENT IS INTENDED TO SERVE AS A CERTIFICATE OF ANALYSIS FOR THE OXYGEN USP WHEN THE RESULTS OF THE PRODUCT TESTING ARE ENTERED BELOW AND THE PRODUCT HAS BEEN DELIVERED TO A REGISTERED OXYGEN USP CUSTOMER. THIS DOCUMENT IS IN COMPLIANCE WITH CURRENT FDA GUIDANCE
TEST SPECIFICATIONS RESULTS
ASSAY GREATER THAN 99.5%
IDENTIFICATION OXYGEN
ODOR NONE
CARBON DIOXIDE LESS THAN 0.03%
CARBON MONOXIDE LESS THAN 0.001%
THE METHODOLOGY USED FOR PERFORMING THE USP TEST FOR ASSAY AND IDENTIFICATION IS THE PARAMAGNETIC ANALYZER MODEL # TELEDYNE 2010 MA. THIS ANALYZER HAS BEEN VALIDATED AS AN ACCEPTABLE ALTERNATIVE TO THE OFFICIAL USP METHOD FOR OXYGEN ASSAY AND IDENTIFICATION. THE VALIDATION STUDY IS AVAILABLE FOR REVIEW UPON REQUEST. ODOR TESTING WAS PERFORMED BY THE OLFACTORY METHOD. SUPPLIER SIGNATURE _________________________ DATE _________________
NUMBER 33 110 A1 - REVISION DATE 05/01/08
FOR ALL OXYGEN USP INSTALLATIONS THE DRIVER SHALL ENSURE AND DOCUMENT THE FOLLOWING: HOSECAPS (PLUGS) IN PLACE PRIOR TO DELIVERY YES/NO, GASKETS ARE SUITABLE FOR USE OR NEW YES/NO, HOSE PURGED PRIOR TO FILLING THE VESSEL YES/NO, HOSE RECAPED (PLUGGED) FOR STORAGE YES/NO. DRIVER SIGNATURE:____________________ DATE:_________________

-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42914-001 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYGEN (UNII: S88TT14065) (OXYGEN - UNII:S88TT14065) OXYGEN 99 L in 100 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42914-001-01 10000000 L in 1 TANK; Type 0: Not a Combination Product 12/15/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205862 12/15/2007 Labeler - Aspen Air U.S., LLC (790650449) Registrant - Aspen Air U.S., LLC (790650449) Establishment Name Address ID/FEI Business Operations Aspen Air U.S., LLC 790650449 manufacture(42914-001)