Label: CETIRIZINE HYDROCHLORIDE tablet
- NDC Code(s): 0781-1683-01, 0781-1683-64
- Packager: Sandoz Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Do not use if you have ever had an allergic reactions to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- •
- if breast-feeding: not recommended
- •
- if pregnant: ask a health professional before use.
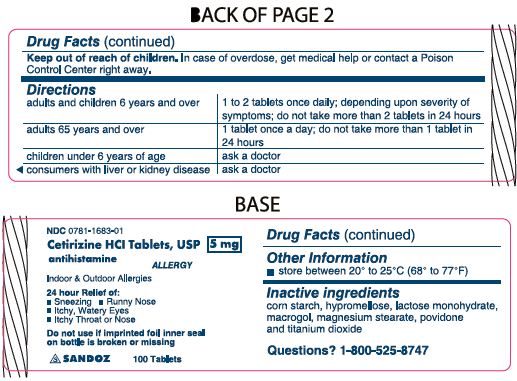
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
adults and children 6 years and over
1 to 2 tablets once daily; depending upon severity of symptoms; do not take more than 2 tablets in 24 hours
adults 65 years and over
1 tablet once a day; do not take more than 1 tablet in 24 hours
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
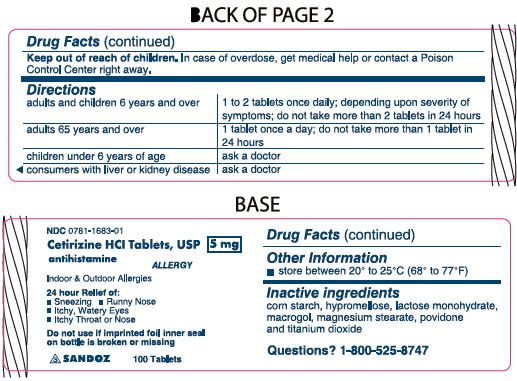
- Inactive ingredients
- Principal Display Panel
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0781-1683 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND (round shape) Size 6mm Flavor Imprint Code SZ;905 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0781-1683-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/27/2007 2 NDC:0781-1683-64 30 in 1 BOX, UNIT-DOSE; Type 0: Not a Combination Product 12/27/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077946 12/27/2007 Labeler - Sandoz Inc (005387188)