MEDICATION GUIDE
ONFI® (ON-fee)
(clobazam)
tablets and oral suspension, C-IV
What is the most important information I should know about ONFI?
-
ONFI is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death. Get emergency help right away if any of the following happens:
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
Do not drive or operate heavy machinery until you know how taking ONFI with opioids affects you.
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including ONFI, which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including ONFI. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take ONFI as prescribed by your healthcare provider.
- Take ONFI exactly as your healthcare provider prescribed.
- Do not share your ONFI with other people.
- Keep ONFI in a safe place and away from children.
-
Physical dependence and withdrawal reactions. ONFI can cause physical dependence and withdrawal reactions.
- Do not suddenly stop taking ONFI. Stopping ONFI suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more ONFI than prescribed or take ONFI for longer than prescribed.
-
ONFI can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how ONFI affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking ONFI without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, ONFI may make your sleepiness or dizziness much worse.
-
Serious skin reactions have been seen when ONFI is taken with other medicines and may require stopping its use. Do not stop taking ONFI without first talking to your healthcare provider.
- A serious skin reaction can happen at any time during your treatment with ONFI, but is more likely to happen within the first 8 weeks of treatment. These skin reactions may need to be treated right away.
- Call your healthcare provider immediately if you have skin blisters, rash, sores in mouth, hives or any other allergic reaction.
-
A serious allergic reaction that may affect your skin or other parts of your body such as your liver, kidneys, heart, or blood cells. This allergic reaction can be life-threatening and can cause death, particularly if it is not treated as early as possible. Call your healthcare provider right away if you have:
- a skin rash
- fever or swollen glands that do not go away
- swelling of your face
- shortness of breath
- dark urine
- yellowing of the skin or whites of the eyes
- Like other antiepileptic medicines, ONFI may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety or irritability
- feeling agitated or restless
- an extreme increase in activity and talking (mania)
- trouble sleeping (insomnia)
- new or worse panic attacks
- acting aggressive, being angry or violent
- acting on dangerous impulses
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What is ONFI?
- ONFI is a prescription medicine used along with other medicines to treat seizures associated with Lennox-Gastaut syndrome in people 2 years of age or older.
- ONFI is a federally controlled substance (C-IV) because it contains clobazam that can be abused or lead to dependence. Keep ONFI in a safe place to prevent misuse and abuse. Selling or giving away ONFI may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
It is not known if ONFI is safe and effective in children less than 2 years old.
Do not take ONFI if you:
- are allergic to clobazam or any of the ingredients in ONFI. See the end of this Medication Guide for a complete list of ingredients in ONFI.
Before you take ONFI, tell your healthcare provider about all your medical conditions, including if you:
- have liver or kidney problems
- have lung problems (respiratory disease)
- have or have had depression, mood problems, or suicidal thoughts or behavior
- use birth control medicine. ONFI may cause your birth control medicine to be less effective. Talk to your healthcare provider about the best birth control method to use.
- are pregnant or plan to become pregnant.
- Taking ONFI late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- Tell your healthcare provider right away if you become pregnant or think you are pregnant while taking ONFI.
- If you become pregnant while taking ONFI, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can register by calling 1-888-233-2334. For more information about the registry go to http://www.aedpregnancyregistry.org. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. ONFI can pass into breast milk.
- Breastfeeding during treatment with ONFI may cause your baby to have sleepiness, feeding problems, and decreased weight gain.
- Talk to your healthcare provider about the best way to feed your baby if you take ONFI.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking ONFI with certain other medicines can cause side effects or affect how well ONFI or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take ONFI?
- Take ONFI exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much ONFI to take and when to take it.
- ONFI tablets can be taken whole, broken in half along the score, or crushed and mixed in applesauce.
- ONFI tablets and oral suspension can be taken with or without food.
- Shake the bottle of ONFI oral suspension well right before you take each dose.
- Measure your dose of ONFI oral suspension using the bottle adapter and dosing syringes that come with your ONFI oral suspension.
- Read the Instructions for Use at the end of this Medication Guide for information on the right way to use ONFI oral suspension.
- Your healthcare provider may change your dose if needed. Do not change your dose of ONFI without talking to your healthcare provider.
- Do not stop taking ONFI without first talking to your healthcare provider.
- Stopping ONFI suddenly can cause serious problems.
- If you take too much ONFI, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking ONFI?
See “What is the most important information I should know about ONFI?”
What are the possible side effects of ONFI?
ONFI may cause serious side effects, including: See "What is the most important information I should know about ONFI?"
The most common side effects of ONFI include:
- sleepiness
- drooling
- constipation
- cough
- pain with urination
- fever
- acting aggressive, being angry, or violent
- difficulty sleeping
- slurred speech
- tiredness
- problems with breathing
These are not all the possible side effects of ONFI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONFI?
- Store ONFI tablets and oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
Tablets
- Keep ONFI tablets in a dry place.
Oral Suspension
- Replace the cap securely after opening.
- Store and dispense the oral suspension in its original bottle in an upright position. Use ONFI oral suspension within 90 days of first opening the bottle.
- After 90 days safely throw away any ONFI oral suspension that has not been used.
- Keep ONFI and all medicines out of the reach of children.
General Information about the safe and effective use of ONFI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ONFI for a condition for which it was not prescribed. Do not give ONFI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ONFI that is written for health professionals.
What are the ingredients in ONFI?
Tablets
Active ingredient: clobazam
Inactive ingredients: modified corn starch, lactose monohydrate, magnesium stearate, silicon dioxide, and talc.
Oral Suspension
Active ingredient: clobazam
Inactive ingredients: magnesium aluminum silicate, xanthan gum, citric acid monohydrate, disodium hydrogen phosphate dihydrate, simethicone emulsion, polysorbate 80, methylparaben, propylparaben, propylene glycol, sucralose, maltitol solution, berry flavor, purified water.
Marketed by: Lundbeck, Deerfield, IL 60015, U.S.A.
ONFI is a registered trademark of Lundbeck
For more information about ONFI, go to www.ONFI.com or call Lundbeck at 1-866-402-8520.

This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 3/2024
Instructions for Use
ONFI® (ON-fee) (clobazam)
Oral Suspension, CIV
Read this Instructions for Use before using ONFI oral suspension and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
Prepare ONFI Oral Suspension Dose
You will need the following supplies: See Figure A
- ONFI oral suspension bottle
- Bottle adapter
- Oral dosing syringe (2 dosing syringes are included in the ONFI oral suspension box).
- Use only 1 syringe to take your dose of ONFI oral suspension. If you lose or damage the syringe, or cannot read the markings, use the other syringe.
Figure A
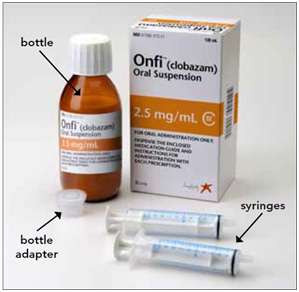
Step 1. Remove the ONFI oral suspension bottle, bottle adapter, and 1 syringe from the box.
Step 2. Shake the bottle well before each use. See Figure B
Figure B

Step 3. Uncap the bottle and firmly insert the bottle adapter into the bottle until the adapter top is even with the bottle top. See Figure C
Figure C
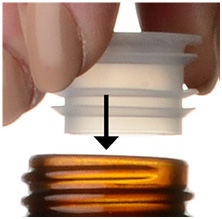
Once the bottle adapter is in place, it should not be removed.
Step 4. Check your dose in milliliters (mL) as prescribed by your healthcare provider. Find this number on the syringe. Do not take more than the prescribed total dose in 1 day. See Figure D
Figure D
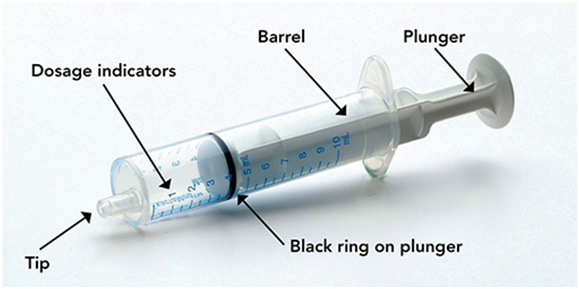
Step 5. Push the plunger all the way down and then insert the syringe into the upright bottle through the opening in the bottle adapter. See Figure E
Figure E

Step 6. With the syringe in place, turn the bottle upside down. Pull the plunger to the number of mLs needed (the amount of liquid medicine in Step 4). See Figure F
Figure F
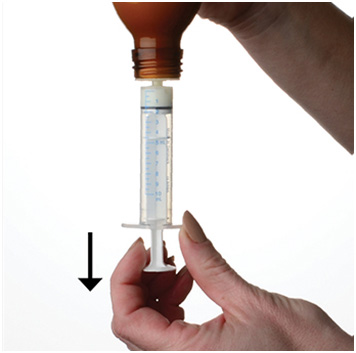
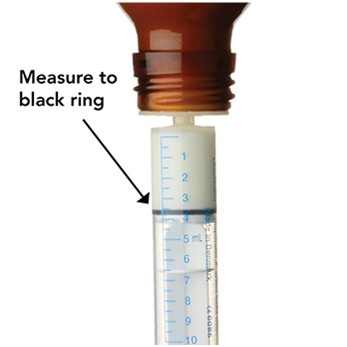
Measure the mLs of medicine using the black ring on the white plunger. See Figure G
Figure G
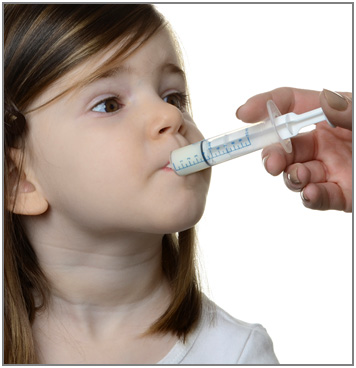
Step 7. Remove the syringe from the bottle adapter. Slowly squirt ONFI oral suspension directly into the corner of your mouth or your child's mouth until all of the liquid medicine in the syringe is given. See Figure H
Figure H
Step 8. Cap the bottle tightly with the adapter in place. If the cap does not fit securely, check to see if the adapter is fully inserted. See Figure I
- Store and dispense ONFI oral suspension in its original bottle in an upright position at 68°F to 77°F (20°C to 25°C).
- Use ONFI oral suspension within 90 days of first opening bottle.
- After 90 days safely throw away any ONFI oral suspension that has not been used.
Figure I

Step 9. Wash the oral syringe after each use.
- To clean the oral syringe, take apart by removing the plunger completely. Pull plunger straight out of the barrel.
- The barrel and plunger can be washed with soap and water, rinsed, and allowed to dry.
- Do not wash the oral syringe in the dishwasher.
This Instruction for Use has been approved by the U.S. Food and Drug Administration.
Marketed by: Lundbeck, Deerfield, IL 60015, U.S.A.

ONFI is a registered trademark of Lundbeck
12/2016