Lamotrigine Extended-Release Tablets
Read this Medication Guide before you start taking lamotrigine extended-release tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about lamotrigine extended-release tablets, ask your healthcare provider or pharmacist.
What is the most important information I should know about lamotrigine extended-release tablets?
1. Lamotrigine extended-release tablets may cause a serious skin rash that may cause you to be hospitalized or to stop lamotrigine extended-release tablets; it may rarely cause death.
There is no way to tell if a mild rash will develop into a more serious reaction. These serious skin reactions are more likely to happen when you begin taking lamotrigine extended-release tablets, within the first 2 to 8 weeks of treatment. But it can happen in people who have taken lamotrigine extended-release tablets for any period of time. Children between 2 to 16 years of age have a higher chance of getting this serious skin reaction while taking lamotrigine. Lamotrigine extended-release tablet is not approved for use in children less than 13 years of age.
The risk of getting a rash is higher if you:
- take lamotrigine extended-release tablets while taking valproate [DEPAKENE® (valproic acid) or DEPAKOTE® (divalproex sodium)].
- take a higher starting dose of lamotrigine extended-release tablets than your healthcare provider prescribed.
- increase your dose of lamotrigine extended-release tablets faster than prescribed.
Lamotrigine extended-release tablets can also cause other types of allergic reactions or serious problems that may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
Call your healthcare provider right away if you have any of the following:
- a skin rash
- hives
- fever
- swollen lymph glands
- painful sores in the mouth or around your eyes
- swelling of your lips or tongue
- yellowing of your skin or eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- severe muscle pain
- frequent infections
These symptoms may be the first signs of a serious skin reaction. A healthcare provider should examine you to decide if you should continue taking lamotrigine extended-release tablets.
2. Like other antiepileptic drugs, lamotrigine extended-release tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Do not stop lamotrigine extended-release tablets without first talking to a healthcare provider.
- Stopping lamotrigine extended-release tablets suddenly can cause serious problems.
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
3. Lamotrigine extended-release tablets may rarely cause aseptic meningitis, a serious inflammation of the protective membrane that covers the brain and spinal cord.
Call your healthcare provider right away if you have any of the following symptoms:
- Headache
- Fever
- Nausea
- Vomiting
- Stiff neck
- Rash
- Unusual sensitivity to light
- Muscle pains
- Chills
- Confusion
- Drowsiness
Meningitis has many causes other than lamotrigine extended-release tablets, which your doctor would check for if you developed meningitis while taking lamotrigine extended-release tablets.
Lamotrigine extended-release tablets can have other serious side effects. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you. Be sure to read the section below entitled "What are the possible side effects of lamotrigine extended-release tablets?"
4. Patients prescribed lamotrigine tablets have sometimes been given the wrong medicine because many medicines have names similar to lamotrigine tablets, so always check that you receive lamotrigine extended-release tablets.
Taking the wrong medication can cause serious health problems. When your healthcare provider gives you a prescription for lamotrigine extended-release tablets:
- Make sure you can read it clearly.
- Talk to your pharmacist to check that you are given the correct medicine.
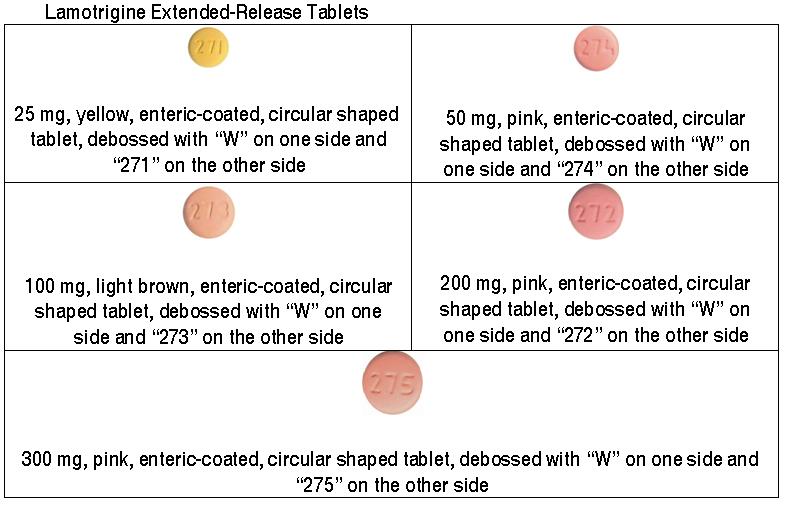
- Each time you fill your prescription, check the tablets you receive against the pictures of the tablets below.
These pictures show the distinct wording, debossing, and sizes of the tablets that help to identify the right strength of lamotrigine extended-release tablets. Immediately call your pharmacist if you receive a lamotrigine extended-release tablet that does not look like one of the tablets shown below, as you may have received the wrong medication.
What is lamotrigine extended-release tablet?
Lamotrigine extended-release tablet is a prescription medicine used:
- together with other medicines to treat partial onset seizures in people 13 years of age and older.
It is not known if lamotrigine extended-release tablet is safe or effective in children less than 13 years of age. Other forms of lamotrigine can be used in children 2 to 12 years.
Who should not take lamotrigine extended-release tablets?
You should not take lamotrigine extended-release tablets if you have had an allergic reaction to lamotrigine or to any of the inactive ingredients in lamotrigine extended-release tablets. See the end of this leaflet for a complete list of ingredients in lamotrigine extended-release tablets.
What should I tell my healthcare provider before taking lamotrigine extended-release tablets?
Before taking lamotrigine extended-release tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have had a rash or allergic reaction to another antiseizure medicine.
- have or have had depression, mood problems or suicidal thoughts or behavior.
- are taking oral contraceptives (birth control pills) or other female hormonal medicines. Do not start or stop taking birth control pills or other female hormonal medicine until you have talked with your healthcare provider. Tell your healthcare provider if you have any changes in your menstrual pattern such as breakthrough bleeding. Stopping these medicines may cause side effects (such as dizziness, lack of coordination, or double vision). Starting these medicines may lessen how well lamotrigine extended-release tablet works.
- are pregnant or plan to become pregnant. It is not known if lamotrigine extended-release tablets will harm your unborn baby. If you become pregnant while taking lamotrigine extended-release tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding. Lamotrigine extended-release tablet passes into breast milk and may cause side effects in a breastfed baby. If you breastfeed while taking lamotrigine extended-release tablets, watch your baby closely for trouble breathing, episodes of temporarily stopping breathing, sleepiness, or poor sucking. Call your baby’s healthcare provider right away if you see any of these problems. Talk to your healthcare provider about the best way to feed your baby if you take lamotrigine extended-release tablets.
Tell your healthcare provider about all the medicines you take or if you are planning to take a new medicine, including prescription and non-prescription medicines, vitamins, and herbal supplements. Using lamotrigine extended-release tablets with certain other medicines can affect each other, causing side effects.
How should I take lamotrigine extended-release tablets?
- Take lamotrigine extended-release tablets exactly as prescribed.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- Do not stop taking lamotrigine extended-release tablets without talking to your healthcare provider. Stopping lamotrigine extended-release tablets suddenly may cause serious problems. For example, if you have epilepsy and you stop taking lamotrigine extended-release tablets suddenly, you may get seizures that do not stop. Talk with your healthcare provider about how to stop lamotrigine extended-release tablets slowly.
- If you miss a dose of lamotrigine extended-release tablets, take it as soon as you remember. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time.
- You may not feel the full effect of lamotrigine extended-release tablets for several weeks.
- If you have epilepsy, tell your healthcare provider if your seizures get worse or if you have any new types of seizures.
- Lamotrigine extended-release tablets can be taken with or without food.
- Do not chew, crush, or divide lamotrigine extended-release tablets.
- Swallow lamotrigine extended-release tablets whole.
- If you have trouble swallowing lamotrigine extended-release tablets, tell your healthcare provider because there may be another form of lamotrigine you can take.
- If you receive lamotrigine extended-release tablets in a blisterpack, examine the blisterpack before use. Do not use if blisters are torn, broken, or missing.
What should I avoid while taking lamotrigine extended-release tablets?
Do not drive a car or operate complex, hazardous machinery until you know how lamotrigine extended-release tablet affects you.
What are possible side effects of lamotrigine extended-release tablets?
- See "What is the most important information I should know about lamotrigine extended-release tablets?"
Common side effects of lamotrigine extended-release tablets include:
- Dizziness
- Tremor
- Double vision
- Nausea
- Vomiting
- Trouble with balance and coordination
- Anxiety
Other common side effects that have been reported with another form of lamotrigine include headache, sleepiness, blurred vision, runny nose, and rash.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of lamotrigine extended-release tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lamotrigine extended-release tablets?
- Store lamotrigine extended-release tablets at room temperature between 20°-25°C (68°-77°F).
- Keep lamotrigine extended-release tablets and all medicines out of the reach of children.
General information about lamotrigine extended-release tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lamotrigine extended-release tablets for a condition for which it was not prescribed. Do not give lamotrigine extended-release tablets to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about lamotrigine extended-release tablets. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about lamotrigine extended-release tablets that is written for healthcare professionals.
For more information, call 1-800-346-6854.
What are the ingredients in lamotrigine extended-release tablets?
Active ingredient: Lamotrigine.
Inactive ingredients: diethyl phthalate, hypromellose, lactose monohydrate, magnesium stearate, methacrylic acid copolymer, polyethylene glycol, talc, titanium dioxide, iron oxide yellow for (25 mg and 100 mg) and iron oxide red for (50 mg, 100 mg, 200 mg and 300 mg).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
DEPAKENE® and DEPAKOTE® are registered trademarks of Abbott Laboratories.
Wockhardt Limited,
Mumbai, India.
Distributed by:
Wockhardt USA LLC.
20 Waterview Blvd.
Parsippany, NJ 07054
USA.
Iss.240812