| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 12/2023 | ||
| Medication
Guide BUTRANS® (BYOO-trans) (buprenorphine) Transdermal System, CIII |
|||
BUTRANS is:
|
|||
Important
information about BUTRANS:
|
|||
Do not use
BUTRANS if you have:
|
|||
Before
applying BUTRANS, tell your healthcare provider if you have a history
of:
|
|
||
Tell your
healthcare provider if you:
|
|||
When using
BUTRANS:
|
|||
While using
BUTRANS DO NOT:
|
|||
| The possible side
effects of BUTRANS are:
constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, itching, redness or rash where the patch is applied. Call your healthcare provider if you have any of these symptoms and they are severe. |
|||
Get emergency
medical help or call 911 right away if you have:
Distributed by: Purdue Pharma L.P., Stamford, CT 06901-3431, www.purduepharma.com or call 1-888-726-7535 |
|||
Instructions
for Use
BUTRANS® (BYOO-trans)
CIII
(buprenorphine)
Transdermal System
Be sure that you read, understand, and follow these Instructions for Use before you use BUTRANS. Talk to your healthcare provider or pharmacist if you have any questions.
- Do not use soap, alcohol, lotions, oils, or other products to remove any leftover adhesive from a patch because this may cause more BUTRANS to pass through the skin.
- Each patch is sealed in its own protective pouch. Do not remove a patch from the pouch until you are ready to use it.
- Do not use a patch if the seal on the protective pouch is broken or if the patch is cut, damaged or changed in any way.
- BUTRANS patches are available in different strengths and patch sizes. Make sure you have the right strength patch that has been prescribed for you.
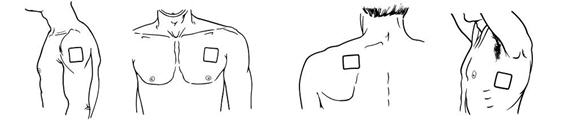
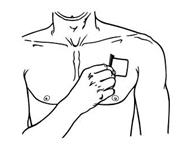
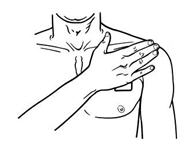
- BUTRANS should be applied to the upper outer arm, upper chest, upper back, or the side of the chest (See Figure A). These 4 sites (located on both sides of the body) provide 8 possible BUTRANS application sites.
Figure A
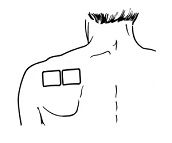
- Do not apply more than 1 patch at the same time unless your doctor tells you to. However, if your healthcare provider tells you to do so, you may use 2 patches as prescribed, applied at the same site (See Figure A for application sites) right next to each other (See Figure B for an example of patch position when applying 2 patches). Always apply and remove the two patches together at the same time.
Figure B
- You should change the skin site where you apply BUTRANS each week, making sure that at least 3 weeks (21 days) pass before you re-use the same skin site.
- Apply BUTRANS to a hairless or nearly hairless skin site. If needed, you can clip the hair at the skin site (See Figure C). Do not shave the area. The skin site should not be irritated. Use only water to clean the application site. You should not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before you apply the patch.
Figure C
- The skin site should be free of cuts and irritation (rashes, swelling, redness, or other skin problems).
- When you apply a new patch, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
- Change the patch at the same time of day, one week (exactly 7 days) after you apply it.
- After removing and disposing of the patch, write down the time it was removed and how it was disposed.
- If you are wearing a patch, remember to remove it before applying a new one.
- Each patch is sealed in its own protective pouch.
- If you are using two patches, remember to apply them at the same site right next to each other. Always apply and remove the two patches together at the same time.
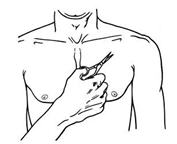
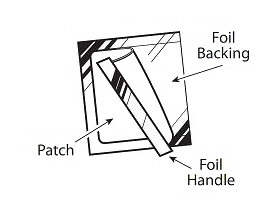
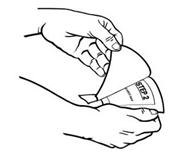
- Use scissors to cut open the pouch along the dotted line (See Figure D) and remove the patch. Do not remove the patch from the pouch until you are ready to use it. Do not use patches that have been cut or damaged in any way.
Figure D
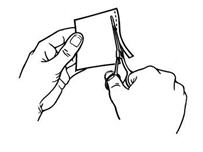
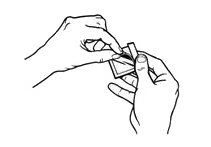
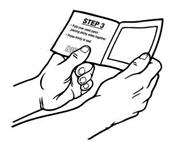
- Hold the patch with the protective liner facing you.
- Gently bend the patch (See Figures E and F) along the faint line and slowly peel the larger portion of the liner, which covers the sticky surface of the patch.
Figure E
Figure F
- Do not touch the sticky side of the patch with your fingers.
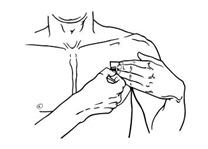
- Using the smaller portion of the protective liner as a handle (See Figure G), apply the sticky side of the patch to one of the 8 body locations described above (See “Where to apply BUTRANS”).
Figure G
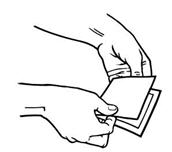
- While still holding the sticky side down, gently fold back the smaller portion of the patch. Grasp an edge of the remaining protective liner and slowly peel it off (See Figure H).
Figure H
- Press the entire patch firmly into place with the palm (See Figure I) of your hand over the patch, for about 15 seconds. Do not rub the patch.
Figure I
- Make sure that the patch firmly sticks to the skin.
- Go over the edges with your fingers to assure good contact around the patch.
- If you are using two patches, follow the steps in this section to apply them right next to each other.
- Always wash your hands after applying or handling a patch.
- After the patch is applied, write down the date and time that the patch is applied. Use this to remember when the patch should be removed.
If the patch falls off right away after applying, throw it away and put a new one on at a different skin site (See “Disposing of BUTRANS Patch”).
If a patch falls off, do not touch the sticky side of the patch with your fingers. A new patch should be applied to a different site. Patches that fall off should not be re-applied. They must be thrown away correctly.
Short-term exposure of the BUTRANS patch to water, such as when bathing or showering, is permitted.
If the edges of the BUTRANS patch start to loosen:
- Apply first aid tape only to the edges of the patch.
- If problems with the patch not sticking continue, cover
the patch with special see-through adhesive dressings (for example
Bioclusive or Tegaderm).
- Remove the backing from the transparent adhesive dressing and place it carefully and completely over the BUTRANS patch, smoothing it over the patch and your skin.
- Never cover a BUTRANS patch with any other bandage or tape. It should only be covered with a special see-through adhesive dressing. Talk to your healthcare provider or pharmacist about the kinds of dressing that should be used.
If your patch falls off later, but before 1 week (7 days) of use, throw it away properly (See “Disposing of a BUTRANS Patch”) and apply a new patch at a different skin site. Be sure to let your healthcare provider know that this has happened. Do not replace the new patch until 1 week (7 days) after you put it on (or as directed by your healthcare provider).
BUTRANS patches should be disposed of by using the Patch-Disposal Unit. Alternatively, the patches can be flushed down the toilet if a drug take-back option is not readily available.
To dispose
of BUTRANS patches in household trash using the Patch-Disposal Unit:
Remove your patch and follow the directions printed on
the Patch-Disposal Unit (See Figure J) or see complete
instructions below. Use one Patch-Disposal Unit for each patch.
Figure J
1. Peel back the disposal unit liner to show the sticky surface (See Figure K).
Figure K
2. Place the sticky side of the used or unused patch to the indicated area on the disposal unit (See Figure L).
Figure L
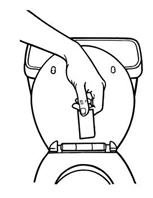
3. Close the disposal unit by folding the sticky sides together (See Figure M). Press firmly and smoothly over the entire disposal unit so that the patch is sealed within.
Figure M
4. The closed disposal unit, with the patch sealed inside may be thrown away in the trash (See Figure N).
Figure N
Do not put expired, unwanted or unused patches in household trash without first sealing them in the Patch-Disposal Unit.
Always remove the leftover patches from their protective pouch and remove the protective liner. The pouch and liner can be disposed of separately in the trash and should not be sealed in the Patch-Disposal Unit.
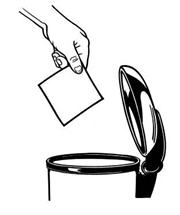
To flush your BUTRANS patches down the toilet:
Remove your BUTRANS patch, fold the sticky sides of a
used patch together and flush it down the toilet right away (See Figure O).
When disposing of unused BUTRANS patches you no longer need, remove the leftover patches from their protective pouch and remove the protective liner. Fold the patches in half with the sticky sides together, and flush the patches down the toilet.
Do not flush the pouch or the protective liner down the toilet. These items can be thrown away in the trash.
If you prefer not to flush the used patch down the toilet, and if there is not a drug take-back option readily available, you must use the Patch-Disposal Unit provided to you to discard the patch.
Never put used BUTRANS patches in the trash without first sealing them in the Patch-Disposal Unit.
This “Instructions for Use” has been approved by the U.S. Food and Drug Administration.
Distributed by: Purdue Pharma L.P., Stamford, CT 06901-3431
Revised: October 2019
©2019, Purdue Pharma L.P.
Bioclusive is a trademark of Systagenix
Wound Management (US), Inc.
Tegaderm is a trademark of
3M.