Medication Guide
Letairis
® (le-TAIR-is)
(ambrisentan) Tablets
|
| Read this Medication Guide before you start taking Letairis and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. |
What is the most important information I should know about Letairis?
-
Serious birth defects.
Letairis can cause serious birth defects if taken during pregnancy.
-
Females must not be pregnant when they start taking Letairis or become pregnant during treatment with Letairis.
- Females who are able to get pregnant must have a negative pregnancy test before beginning treatment with Letairis, each month during treatment with Letairis, and one month after stopping Letairis. Talk to your doctor about your menstrual cycle. Your doctor will decide when to do a pregnancy test, and will order a pregnancy test for you depending on your menstrual cycle.
- Females who
are able to get pregnant are females who:
- have entered puberty, even if they have not started their menstrual period,
and
- have a uterus,
and
- have not gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed.
- Females who
are not able to get pregnant are females who:
- have not yet entered puberty,
or
- do not have a uterus,
or
- have gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed,
or
- who are infertile for any other medical reason and this infertility is permanent and cannot be reversed
Females who are able to get pregnant must use two acceptable forms of birth control during treatment with Letairis, and for one month after stopping Letairis because the medicine may still be in the body.
- If you have had a tubal sterilization or have an IUD (intrauterine device) or progesterone implant, these methods can be used alone and no other form of birth control is needed.
- Talk with your doctor or gynecologist (a doctor who specializes in female reproduction) to find out about options for acceptable forms of birth control that you may use to prevent pregnancy during treatment with Letairis.
- If you decide that you want to change the form of birth control that you use, talk with your doctor or gynecologist to be sure that you choose another acceptable form of birth control.
See the chart below for Acceptable Birth Control Options during treatment with Letairis.
Acceptable Birth Control Options
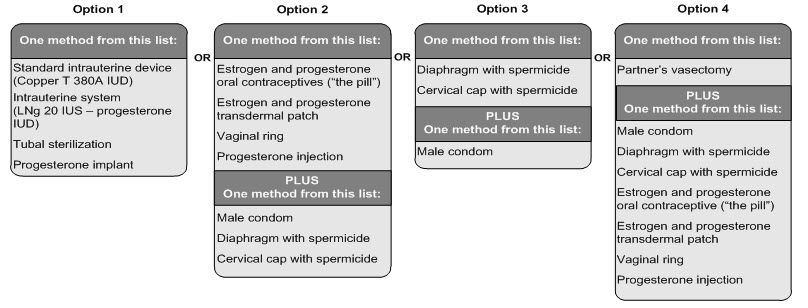
-
Do not have unprotected sex. Talk to your doctor or pharmacist right away if you have unprotected sex or if you think your birth control has failed. Your doctor may tell you to use emergency birth control.
-
Tell your doctor right away if you miss a menstrual period or think you may be pregnant for any reason.
If you are the parent or caregiver of a female child who started taking Letairis before reaching puberty, you should check your child regularly to see if she is developing signs of puberty. Tell your doctor right away if you notice that she is developing breast buds or pubic hair. Your doctor should decide if your child has reached puberty
. Your child may reach puberty before having her first menstrual period.
Females can only receive Letairis through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Ambrisentan REMS. If you are a female who can get pregnant, you must talk to your doctor, understand the benefits and risks of Letairis, and agree to all of the instructions in the Ambrisentan REMS.
Males can receive Letairis without taking part in the Ambrisentan REMS.
|
What is Letairis?
- Letairis is a prescription medicine used to treat pulmonary arterial hypertension (PAH), which is high blood pressure in the arteries of your lungs.
- Letairis can improve your ability to exercise and it can help slow down the worsening of your physical condition and symptoms.
- When taken with tadalafil, Letairis is used to reduce the risk of your disease progressing, to reduce the risk of hospitalization due to worsening pulmonary arterial hypertension (PAH), and to improve your ability to exercise.
- It is not known if Letairis is safe and effective in children.
|
Who should not take Letairis?
Do not take Letairis if:
-
you are pregnant, plan to become pregnant, or become pregnant during treatment with Letairis. Letairis can cause serious birth defects. (See
"
What is the most important information I should know about Letairis?"
)
Serious birth defects from Letairis happen early in pregnancy.
- you have a condition called Idiopathic Pulmonary Fibrosis (IPF)
|
What should I tell my doctor before taking Letairis?
Before you take Letairis, tell your doctor if you:
- have been told that you have a low red blood cell level (anemia)
- have liver problems
- have any other medical conditions
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Letairis and other medicines may affect each other, causing side effects. Do not start any new medicines until you check with your doctor.
Especially tell your doctor if you take the medicine cyclosporine (Gengraf, Neoral, Sandimmune). Your doctor may need to change your dose of Letairis.
|
How should I take Letairis?
- Letairis will be mailed to you by a certified pharmacy. Your doctor will give you complete details.
- Take Letairis exactly as your doctor tells you to take it. Do not stop taking Letairis unless your doctor tells you to stop.
- You can take Letairis with or without food.
- Do not split, crush or chew Letairis tablets.
- It will be easier to remember to take Letairis if you take it at the same time each day.
- If you take more than your regular dose of Letairis, call your doctor right away.
- If you miss a dose, take it as soon as you remember that day. Take your next dose at the regular time. Do not take two doses at the same time to make up for a missed dose.
|
What should I avoid while taking Letairis?
-
Do not get pregnant while taking Letairis. (See the serious birth defects section of the Medication Guide above called
"
What is the most important information I should know about Letairis?"
) If you miss a menstrual period, or think you might be pregnant, call your doctor right away.
-
It is not known if Letairis passes into your breast milk. You should not breastfeed if you are taking Letairis. Talk to your doctor about the best way to feed your baby if you take Letairis.
|
What are the possible side effects of Letairis?
Letairis can cause serious side effects including:
- See
"
What is the most important information I should know about Letairis?"
-
Swelling all over the body (fluid retention) can happen within weeks after starting Letairis. Tell your doctor right away if you have any unusual weight gain, tiredness, or trouble breathing while taking Letairis. These may be symptoms of a serious health problem. You may need to be treated with medicine or need to go to the hospital.
-
Decreased sperm count. Decreased sperm counts have happened in some men taking a medicine that is like Letairis. A decreased sperm count may affect the ability to father a child. Tell your doctor if being able to have children is important to you.
-
Low red blood cell levels (anemia) can happen during the first weeks after starting Letairis. If this happens, you may need a blood transfusion. Your doctor will do blood tests to check your red blood cells before starting Letairis. Your doctor may also do these tests during treatment with Letairis.
The most common side effects of Letairis include: |
- swelling of hands, legs, ankles and feet (peripheral edema)
- stuffy nose (nasal congestion)
|
- inflamed nasal passages (sinusitis)
- hot flashes or getting red in the face (flushing)
|
| Some medicines that are like Letairis can cause liver problems. Tell your doctor if you get any of these symptoms of a liver problem while taking Letairis: |
- loss of appetite
- nausea or vomiting
- fever
- achiness
|
- generally do not feel well
- pain in the upper right stomach (abdominal) area
- yellowing of your skin or the whites of your eyes
- dark urine
- itching
|
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Letairis. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
How should I store Letairis?
Store Letairis at room temperature between 68°F to 77°F (20°C to 25°C), in the package it comes in.
Keep Letairis and all medicines out of the reach of children. |
General information about the safe and effective use of Letairis
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Letairis for a condition for which it was not prescribed. Do not give Letairis to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Letairis. If you would like more information, ask your doctor. You can ask your doctor or pharmacist for information about Letairis that is written for health professionals.
|
What are the ingredients in Letairis?
Active ingredient: ambrisentan
Inactive Ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The tablets are film-coated with a coating material containing FD&C Red #40 aluminum lake, lecithin, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Gilead Sciences, Inc., Foster City, CA 94404
Letairis is a registered trademark of Gilead Sciences, Inc. Gilead and the Gilead logo are trademarks of Gilead Sciences, Inc. Other brands noted herein are the property of their respective owners.
© 2019 Gilead Sciences, Inc.
GS22-081-017
For more information call 1-866-664-5327 or go to www.letairis.com or www.gilead.com.
|
