-
-
MEDICATION GUIDE
|
-
-
PROMACTA®(pro-MAC-ta)
|
-
-
PROMACTA®(pro-MAC-ta)
|
-
-
(eltrombopag)
|
-
-
(eltrombopag)
|
-
-
tablets
|
-
-
for oral suspension
|
-
- Read this Medication Guide before you start taking PROMACTA and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or treatment.
|
-
-
What is the most important information I should know about PROMACTA?
-
- PROMACTA can cause serious side effects, including:
-
-
Liver problems. If you have chronic hepatitis C virus, and take PROMACTA with interferon and ribavirin treatment, PROMACTA may increase your risk of liver problems. Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems:
- •
- yellowing of the skin or the whites of the eyes (jaundice)
- •
- unusual darkening of the urine
- •
- unusual tiredness
- •
- right upper stomach area (abdomen) pain
- •
- confusion
- •
- swelling of the stomach area (abdomen)
-
-
See “What are the possible side effects of PROMACTA?” for other side effects of PROMACTA.
|
-
-
What is PROMACTA?
-
- PROMACTA is a prescription medicine used to treat adults and children 1 year of age and older with low blood platelet counts due to chronic immune (idiopathic) thrombocytopenia (ITP), when other medicines to treat ITP or surgery to remove the spleen have not worked well enough.
-
- PROMACTA is also used to treat patients with:
- •
- low blood platelet counts due to chronic hepatitis C virus (HCV) infection before and during treatment with interferon.
- •
- severe aplastic anemia (SAA) when other medicines to treat SAA have not worked well enough.
-
- PROMACTA is used to try to raise platelet counts in order to lower your risk for bleeding.
-
- PROMACTA is not used to make platelet counts normal.
-
- PROMACTA is for treatment of certain people with low platelet counts caused by chronic ITP, chronic HCV, or SAA, not low platelet counts caused by other conditions or diseases.
-
- It is not known if PROMACTA is safe and effective when used with other antiviral medicines that are approved to treat chronic hepatitis C.
-
- It is not known if PROMACTA is safe and effective in children with chronic hepatitis C or severe aplastic anemia or in children younger than 1 year with ITP.
|
-
-
What should I tell my healthcare provider before taking PROMACTA?
-
-
Before you take PROMACTA, tell your healthcare provider if you:
- •
- have liver or kidney problems
- •
- have or had a blood clot
- •
- have a history of cataracts
- •
- have had surgery to remove your spleen (splenectomy)
- •
- have bleeding problems
- •
- are Asian and you are of Chinese, Japanese, Taiwanese, or Korean ancestry. You may need a lower dose of PROMACTA.
- •
- have any other medical conditions
- •
- are pregnant or plan to become pregnant. It is not known if PROMACTA will harm an unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if PROMACTA passes into your breast milk. You and your healthcare provider should decide whether you will take PROMACTA or breastfeed. You should not do both.
-
-
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. PROMACTA may affect the way certain medicines work. Certain other medicines may affect the way PROMACTA works.
-
- Especially tell your healthcare provider if you take:
- •
- certain medicines used to treat high cholesterol, called “statins”.
- •
- a blood thinner medicine.
-
- Certain medicines may keep PROMACTA from working correctly. Take PROMACTA at least 2 hours before or 4 hours after taking these products:
- •
- antacid medicine used to treat stomach ulcers or heartburn
- •
- multivitamins or products that contain iron, calcium, aluminum, magnesium, selenium, and zinc which may be found in mineral supplements
-
- Ask your healthcare provider if you are not sure if your medicine is one that is listed above.
-
- Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
|
-
-
How should I take PROMACTA?
- •
- Take PROMACTA exactly as your healthcare provider tells you to take it. Your healthcare provider will prescribe the dose of PROMACTA tablets or PROMACTA oral suspension that is right for you.
- •
- If your healthcare provider prescribes PROMACTA oral suspension, see “Instructions for Use” that comes with your medicine for instructions on how to prepare and take your dose.
- •
- Do not stop taking PROMACTA without talking with your healthcare provider first. Do not change your dose or schedule for taking PROMACTA unless your healthcare provider tells you to change it.
- •
- Take PROMACTA on an empty stomach, either 1 hour before or 2 hours after eating food.
- •
- Take PROMACTA at least 2 hours before or 4 hours after eating dairy products and calcium-fortified juices.
- •
-
Take PROMACTA tablets whole. Do not crush PROMACTA tablets and mix with food or liquids.
-
- If you miss a dose of PROMACTA, wait and take your next scheduled dose. Do not take more than one dose of PROMACTA in one day.
- •
- If you take too much PROMACTA, you may have a higher risk of serious side effects. Call your healthcare provider right away.
- •
- Your healthcare provider will check your platelet count during your treatment with PROMACTA and change your dose of PROMACTA as needed.
- •
- Tell your healthcare provider about any bruising or bleeding that happens while you take and after you stop taking PROMACTA.
|
-
-
What should I avoid while taking PROMACTA?
-
- Avoid situations and medicines that may increase your risk of bleeding.
|
-
-
What are the possible side effects of PROMACTA?
-
-
PROMACTA may cause serious side effects, including:
- •
- See “What is the most important information I should know about PROMACTA?”
- •
-
Abnormal liver function tests. Your healthcare provider will order blood tests to check your liver before you start taking PROMACTA and during your treatment. In some cases treatment with PROMACTA may need to be stopped due to changes in your liver function tests.
- •
-
High platelet counts and higher risk for blood clots. Your risk of getting a blood clot is increased if your platelet count is too high during treatment with PROMACTA. Your risk of getting a blood clot may also be increased during treatment with PROMACTA if you have normal or low platelet counts. You may have severe problems or die from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your healthcare provider will check your blood platelet counts, and change your dose or stop PROMACTA if your platelet counts get too high. Tell your healthcare provider right away if you have signs and symptoms of a blood clot in the leg, such as swelling, pain, or tenderness in your leg.
-
- People with chronic liver disease may be at risk for a type of blood clot in the stomach area. Tell your healthcare provider right away if you have stomach area pain that may be a symptom of this type of blood clot.
- •
-
New or worsened cataracts (a clouding of the lens in the eye). New or worsened cataracts have happened in people taking PROMACTA. Your healthcare provider will check your eyes before and during your treatment with PROMACTA. Tell your healthcare provider about any changes in your eyesight while taking PROMACTA.
|
-
-
The most common side effects of PROMACTA in adults when used to treat chronic ITP are:
- •
- nausea
- •
- diarrhea
- •
- upper respiratory tract infection. Symptoms may include runny nose, stuffy nose, and sneezing
- •
- vomiting
- •
- muscle aches
- •
- urinary tract infection. Symptoms may include frequent or urgent need to urinate, low fever in some people, pain or burning with urination.
- •
- pain or swelling (inflammation) in your throat or mouth (oropharyngeal pain and pharyngitis)
- •
- abnormal liver function tests
- •
- back pain
- •
- ”flu”-like symptoms (influenza) including fever, headache, tiredness, cough, sore throat, and body aches
- •
- skin tingling, itching, or burning
- •
- rash
-
-
The most common side effects of PROMACTA in children 1 year and older when used to treat chronic ITP are:
- •
- upper respiratory tract infection. Symptoms may include runny nose, stuffy nose, and sneezing.
- •
- pain or swelling (inflammation) in your nose or throat (nasopharyngitis)
- •
- cough
- •
- diarrhea
- •
- fever
- •
- runny, stuffy nose (rhinitis)
- •
- stomach (abdominal) pain
- •
- pain or swelling (inflammation) in your throat or mouth (oropharyngeal pain)
- •
- toothache
- •
- rash
- •
- abnormal liver function tests
-
-
The most common side effects when PROMACTA is used in combination with other medicines to treat chronic HCV are:
- •
- low red blood cell count (anemia)
- •
- fever
- •
- tiredness
- •
- headache
- •
- nausea
- •
- diarrhea
- •
- decreased appetite
- •
- “flu”-like symptoms (influenza) including fever, headache, tiredness, cough, sore throat, and body aches
- •
- feeling weak
- •
- trouble sleeping
- •
- cough
- •
- itching
- •
- chills
- •
- muscle aches
- •
- hair loss
- •
- swelling in your ankles, feet, and legs
-
-
The most common side effects when PROMACTA is used to treat severe aplastic anemia are:
- •
- nausea
- •
- feeling tired
- •
- cough
- •
- diarrhea
- •
- headache
- •
- pain in arms, legs, hands, or feet
- •
- shortness of breath
- •
- fever
- •
- dizziness
- •
- pain in the nose or throat
- •
- abdominal pain
- •
- bruising
- •
- muscle spasms
- •
- abnormal liver function tests
- •
- joint pain
- •
- runny nose
-
- Laboratory tests may show abnormal changes to the cells in your bone marrow.
-
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of PROMACTA. For more information, ask your healthcare provider or pharmacist.
-
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
|
-
-
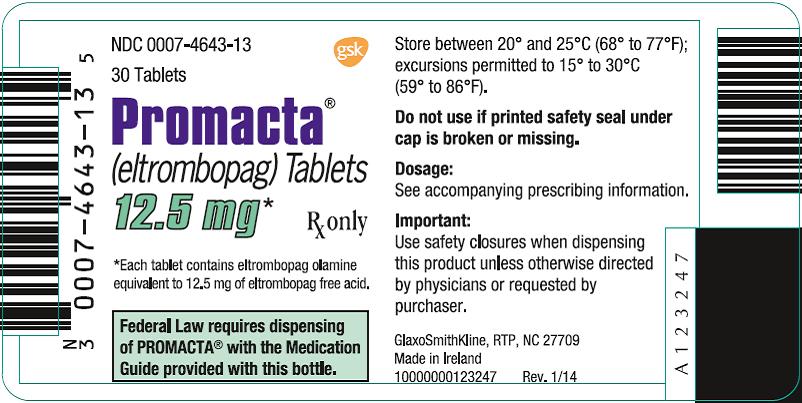
How should I store PROMACTA tablets and oral suspension?
-
-
Tablets:
- •
- Store PROMACTA tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep PROMACTA tightly closed in the bottle given to you.
- •
- The PROMACTA bottle may contain a desiccant pack to help keep your medicine dry. Do not remove the desiccant pack from the bottle.
-
-
For oral suspension:
- •
- Store PROMACTA for oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- After mixing, PROMACTA should be taken right away but may be stored for no more than 30 minutes between 68°F to 77°F (20°C to 25°C). Throw away (discard) the mixture if not used within 30 minutes.
-
-
Keep PROMACTA and all medicines out of the reach of children.
|
-
-
General information about the safe and effective use of PROMACTA
-
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PROMACTA for a condition for which it was not prescribed. Do not give PROMACTA to other people, even if they have the same symptoms that you have. It may harm them.
-
- This Medication Guide summarizes the most important information about PROMACTA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about PROMACTA that is written for health professionals.
-
- For more information about PROMACTA, go to www.PROMACTA.com or call 1-888-825-5249.
|
-
-
What are the ingredients in PROMACTA?
-
-
Tablets:
-
-
Active ingredient: eltrombopag olamine.
-
-
Inactive ingredients:
- •
-
Tablet Core: magnesium stearate, mannitol, microcrystalline cellulose, povidone, and sodium starch glycolate.
- •
-
Coating: hypromellose (12.5-mg, 25-mg, 50-mg, and 75-mg tablets) or polyvinyl alcohol and talc (100-mg tablet), polyethylene glycol 400, titanium dioxide, polysorbate 80 (12.5-mg tablet), and FD&C Yellow No. 6 aluminum lake (25-mg tablet), FD&C Blue No. 2 aluminum lake (50-mg tablet), Iron Oxide Red and Iron Oxide Black (75-mg tablet), or Iron Oxide Yellow and Iron Oxide Black (100-mg tablet).
-
-
For oral suspension:
-
-
Active ingredient: eltrombopag olamine.
-
-
Inactive ingredients: mannitol, sucralose, xanthan gum.
-
- PROMACTA is a registered trademark of the GSK group of companies. GlaxoSmithKline Research Triangle Park, NC 27709
-
- ©2015, the GSK group of companies. All rights reserved. PRM:10MG
|