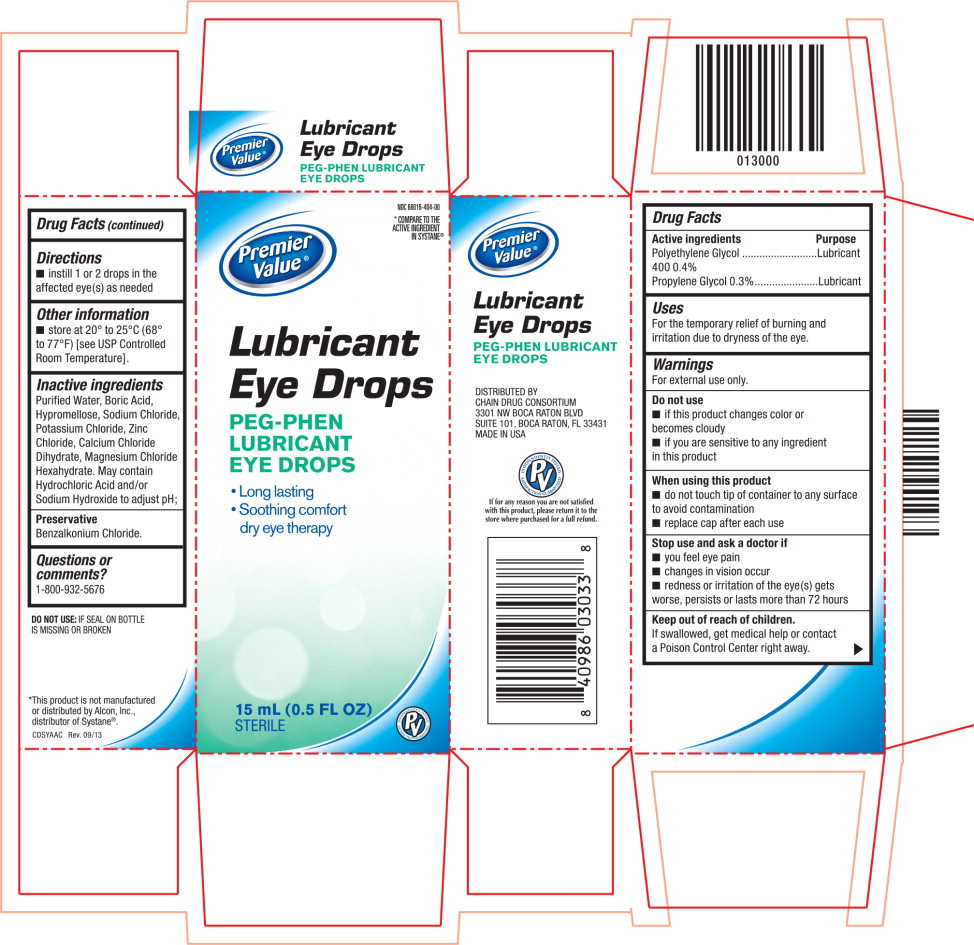
LUBRICANT EYE DROPS- polyethylene glycol 400 and propylene glycol solution/ drops
Chain Drug Consortium
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Inactive ingredients
Purified Water, Boric Acid, Hypromellose, Sodium Chloride, Potassium Chloride, Zinc Chloride, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate. May contain Hydrochloric Acid and/or Sodium Hydroxide to adjust pH;
| LUBRICANT EYE DROPS
polyethylene glycol 400 and propylene glycol solution/ drops |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
Revised: 10/2020
Document Id: 1e590137-4b58-42fa-96bb-277ec585358a
Set id: ffe98484-20b7-4578-b9cc-0707f8e3d2b1
Version: 3
Effective Time: 20201021
Chain Drug Consortium