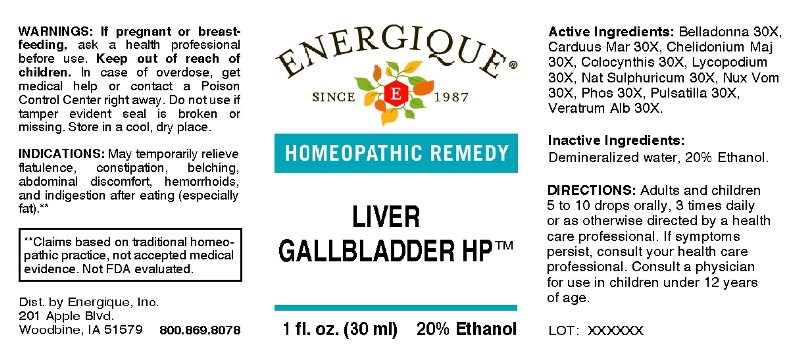
LIVER GALLBLADDER HP- belladonna, carduus marianus, chelidonium majus, colocynthis, lycopodium clavatum, natrum sulphuricum, nux vomica, phosphorus, pulsatilla, veratrum album liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Belladonna 30X, Carduus Marianus 30X, Chelidonium Majus 30X, Colocynthis 30X, Lycopodium Clavatum 30X, Natrum Sulphuricum 30X, Nux Vomica 30X, Phosphorus 30X, Pulsatilla 30X, Veratrum Album 30X.
INDICATIONS:
May temporarily relieve flatulence, constipation, belching, abdominal discomfort, hemorrhoids and indigestion after eating (especially fat).**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist, consult your health care professional. Consult a physician for use in children under 12 years of age.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
| LIVER GALLBLADDER
HP
belladonna, carduus marianus, chelidonium majus, colocynthis, lycopodium clavatum, natrum sulphuricum, nux vomica, phosphorus, pulsatilla, veratrum album liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apothea Company | 844330915 | manufacture(44911-0010) , api manufacture(44911-0010) , label(44911-0010) , pack(44911-0010) | |